Abstract
Background:
Guidelines for depth of chest compressions in pediatric cardiopulmonary resuscitation (CPR) are based on sparse evidence.
Objective:
We sought to evaluate the performance of the two most widely recommended chest compression depth levels for pediatric CPR (1.5 inches and 1/3 the anterior-posterior diameter-APd) in a controlled swine model of asphyxial cardiac arrest.
Methods:
We executed a 2-group, randomized laboratory study with an adaptive design allowing early termination for overwhelming injury or benefit. Forty mixed-breed domestic swine (mean weight = 26kg) were sedated, anesthetized and paralyzed along with endotracheal intubation and mechanical ventilation. Asphyxial cardiac arrest was induced with fentanyl overdose. Animals were untreated for 9 minutes followed by mechanical CPR with a target depth of 1.5 inches or 1/3 the APd. Advanced life support drugs were administered IV after 4 minutes of basic resuscitation followed by defibrillation at 14 minutes. The primary outcomes were return of spontaneous circulation (ROSC), hemodynamics and CPR-related injury severity.
Results:
Enrollment in the 1/3 APd group was stopped early due to overwhelming differences in injury. Twenty-three animals were assigned to the 1.5 inch group and 15 assigned to the 1/3 APd group, per an adaptive group design. The 1/3 APd group had increased frequency of rib fracture (6.7 vs 1.7, p<0.001) and higher proportions of several anatomic injury markers than the 1.5 inch group, including sternal fracture, hemothorax and blood in the endotracheal tube (p<0.001). ROSC and hemodynamic measures were similar between groups.
Conclusion:
In this pediatric model of cardiac arrest, chest compressions to 1/3APd were more harmful without a concurrent benefit for resuscitation outcomes compared to the 1.5 inch compression group.
Keywords: Out-of-hospital cardiac arrest, pediatric, chest compressions, injury, preclinical
Introduction
Although out-of-hospital cardiac arrest (OHCA) in children is relatively uncommon compared with adult (1.1 and 9.5 per 100,000 versus 140.7 per 100,000 annually in the United States), it is a leading cause of childhood death.1 Thus, development of effective methods of resuscitating children is important, especially as the cumulative loss of life, disability, and productivity is potentially greater for younger patients.2 In addition, survival outcomes are generally superior in children with the exception of infants with sudden infant death syndrome.3–5
Current guidelines governing cardiopulmonary resuscitation (CPR) for adults is informed by a large body of evidence derived from clinical prospective and retrospective studies as well as basic and translational science.6 These data demonstrate the significance of the mechanical parameters of chest compressions, including the rate (defining the number of chest compressions delivered in a minute) and the depth (defining the internal displacement of the chest) at which chest compressions are delivered.
Conversely, pediatric CPR guidelines are largely consensus-based or are extrapolations from adult evidence. The American Heart Association guidelines for pediatric resuscitation include depth recommendations for either one-third the anterior-posterior chest diameter (1/3 APd) or equivalently 1.5 inches.7 It is unclear whether either depth target is relatively advantageous, although it is reasonable to assume that a proportional depth target would provide more consistent injury effects across varying body types compared to a fixed depth target, given that chest dimensions across development are not equally proportioned. Furthermore, retrospective clinical studies of pediatric anatomical data indicate that the absolute measures corresponding to 1/3 APd diverge significantly from 1.5 inches as children age, suggesting the potential for disparate survival benefits and injury characteristics occurring with these targets.8 9 However, there are no studies directly comparing benefits and harms of 1/3 APd versus 1.5 inch targets.
In the present study, we sought to investigate and compare the performance of 1/3 APd and 1.5 inch depth targets in three domains: return of spontaneous circulation (ROSC), hemodynamics, and CPR-related injury. We employed an established swine model of asphyxial arrest with concurrent opioid overdose to test our hypotheses. Owing to the observation that swine in our laboratory typically have an APd greater than 6 inches, yielding 1/3 APd measurements substantially greater than 1.5 inches, we hypothesized that ROSC, hemodynamics and injury rates would differ between the two target depths.
Methods
This experimental randomized laboratory study was approved by our Institutional Animal Care and Use Committee conducted in accordance with the Guide for Care and Use of Laboratory Animals.
Anesthesia and Surgical Instrumentation
We used mixed breed Yorkshire swine of either sex, with a mean mass of 26 kgs. This size approximates the 50th percentile for a seven year old child, and the 10th percentile for a 10 year old child. We sedated the animals with ketamine (10 mg/kg; Ketaset, Zoetis LLC, USA) and xylanzine (4 mg/kg; Covetrus, Inc, USA) administered via intramuscular injection. When animals were fully sedated, they were moved into a supine position by the surgical team and their AP diameter was measured using an adjustable 90-degree T-square. We then established an ear vein intravenous (IV) line for administration of an initial anesthetic fentanyl bolus (50 ug/kg; Hikma Pharmaceuticals PLC, USA), followed by continuous maintenance infusion (30 ug/kg/hr) run on an mechanical syringe pump (BS-300, Braintree Scientific, Inc., USA). We then induced paralysis with vecuronium (5mg IV; McKesson Medical-Surgical, Inc., USA), intubated the animals, and began mechanical ventilation (PUMA V6, DRE Veterinary, Inc., USA) with a rate titrated to achieve eucapnia (12 – 16 BrPM), a tidal volume of 10mL/kg, and a inspiratio:expiration ratio of 40%. Invasive pressure transducers (SPR-350S, Millar, Inc., USA) were placed in the aorta (central arterial pressure – CAP) and right atrium (central venous pressure – CVP), and the right common carotid artery was instrumented with a laboratory doppler flow sensor (MA3PSB, Transonic Systems, Inc., USA). Coronary perfusion pressure was calculated by taking the arithmetic difference between the CAP and CVP waveforms and then averaging 50 samples from the end-diastolic phase of the compression cycle. All physiologic signals, including pulse oximetry, capnography and multi-lead electrocardiogram (ECG) were monitored and recorded continuously throughout the experiment using a commercial analog-to-digital data acquisition platform (PowerLab 16/30, ADInstruments, Inc., Australia).
Experimental Procedure
Following instrumentation, we conducted an arterial blood gas and electrolyte measurement using a portable clinical analyzer (iSTAT-300, Abbott Point of Care, Inc., USA). We then administered in sequence through the ear vein IV line a rapid bolus of vecuronium (10 mg), followed by a bolus of fentanyl (30 ug/kg) delivered over a period of 2 minutes. The ventilator was then disconnected from the airway and the endotracheal tube was occluded. The animals were untreated for 9 minutes to simulate the time required to recognize the cardiac arrest, call 9-1-1 and the EMS response interval, and to allow for mechanical cardiac function to cease. At the end of this interval, animals were assigned to either of the chest compression depth groups (described below), and chest compressions were initialized with a custom made servomotor-driven CPR device.10 This device, while capable of using biosignal feedback, was used in simple fixed-rate, fixed depth mode for this series. The device was configured for a 50% duty cycle and utilized an up/down stroke velocity in our observation comparable to commercial devices. The device did not have a force limiter, and as such proceeded to the target depth without regard to the opposing force it encountered. Structurally, the device utilized a repurposed frame from a Thumper chest compression device, which situates the piston on a rotating arm fixed to a pylon that sits to the side of the subject. The piston was rotated into position above the sternum, lowered to achieve contact surface contact between the sternum and piston head, and then locked in place with a tension bolt. The device used a solid plastic piston head dampened with a rubberized pad, both repurposed and modified from a commercial chest compression devices (LUCAS 2, Stryker, Inc, USA) so as to not create suction on the upstroke. Asynchronous ventilations were delivered mechanically with 100% O2 at the final pre-arrest rate and tidal volumes. After 4 minutes of basic CPR, we administered epinephrine (0.1mg/kg; Par Pharmaceutical, USA) and sodium bicarbonate (1mEq/kg; Pfizer, Inc., USA) through the femoral venous introducer port. Beginning at 5 minutes of CPR, compressions were paused every 2 minutes for rhythm analysis and if indicated by the presence of ventricular fibrillation (VF) defibrillation was attempted with an energy of 150J, reflecting an empirically determine laboratory optimum, using a standard clinical defibrillator-monitor (M-Series, ZOLL Medical Corporation, USA). Resuscitation efforts continued for up to 20 minutes or until ROSC was achieved. ROSC was defined as an organized ECG rhythm coupled with pulses present in the arterial pressure waveform in the absence of chest compressions and a mean arterial pressure exceeding 40 mmHg. Animals achieving initial ROSC were observed for 30 minutes and then euthanized via administration of KCl (40 mEq; Fresenius SE, Germany) delivered IV. Throughout each experiment, critical events were recorded with timestamps to enable calculation of key experimental intervals, including time from airway occlusion to pulselessness and time from start of CPR to ROSC.
Chest Compression Depth Groups
Figure 1 illustrates the experimental design, including the chest compression depth group parameters. Animals in Group 1 received chest compressions at a rate of 100 compressions per minute (CPM) at a depth of 1.5 inches, while animals in Group 2 received chest compressions at a depth of 1/3 APd. Randomization order was determined via computer generated list prior to beginning the study.
Figure 1. Experimental Timeline –
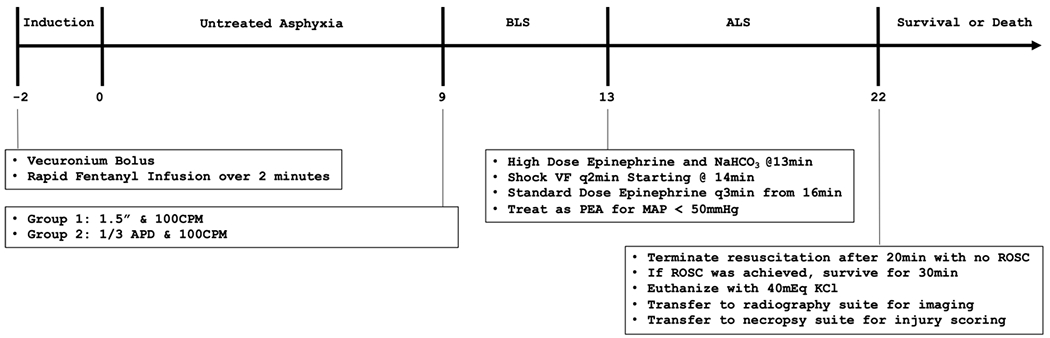
The complete experimental timeline, including experimental groups and associated threatments is shown on a timeline starting at administration of the arrest induction cocktail.
Abbreviations: ALS – Advanced Life Support; BLS – Basic Life Support; CPM – Compressions Per Minute; MAP – Mean Arterial Pressure; PEA – Pulseless Electrical Activity; ROSC – Return of Spontaneous Circulation; VF – Ventricular Fibrillation
Post-Mortem Examination
Immediately following euthanasia or cessation of unsuccessful resuscitation, necropsy was performed by a pair of examiners from a pool of qualified research veterinarians and veterinary technicians who were blinded to group assignment. The pair of examiners always comprised one veterinarian and one veterinary technician who used a standardized, multipoint injury scoring system, including presence of blood in the ET tube, number and location of rib fractures, sternal fracture or displacement, hemothorax, aortic dissection, vena caval dissection, myocardial contusion, and liver/spleen contusion. The lungs were also examined and each lobe was scored a 0 if no injury was apparent, 1 if <= 50% of the surface area showed injury, or 2 if >= 50% of surface area showed injury. Thus, the lung injury score could range from zero to ten. The total lung injury score was taken as the sum of the two examiners’ injury scores and could range from zero to twenty.
A limited number of lateral thoracic x-ray images were taken from a subset of animals for exploratory analysis of the total compressible depth of the chest, which differs from the APd as it reflects the internal distance between the sternum and spine. Xray images were recorded digitally and imported into MATLAB (r2019a, Mathworks, Inc, Nattick, MA) in native DICOM format. The postmortem APd and the accompanying intrathoracic diameter were measured using the manual drawline tool using the Imager Pixel Spacing metadata field to convert the measurements to inches. Compressibility of the chest was calculated as the ratio of the intrathoracic diameter to the overall APd.
Sample Size
The study was powered to detect an 11.3 mmHg difference in CPP between groups during the ALS phase of resuscitation with 85% power. Following on a consistent relationship between ROSC and CPP in our laboratory and owing to the futility of a resuscitation method that would result in fatal injuries, we designed an interim analysis to determine termination of enrollment in any group on the outcomes of ROSC and unsurvivable injury using a treatment toxicity metric. With a total possible enrollment of 28 animals per group, interim analyses were conducted after accumulation of 18 and 22 animals per group.
Analysis
The primary outcomes were hemodynamics (CAP, CPP and CBF), ROSC, and injury severity.
The primary safety outcome was unsurvivable injury (i.e. toxicity), which we defined as either a total lung injury score ≤16 between both necropsy reviewers plus presence of hemothorax (needed to be combination of both), or disruption of either the aorta or vena cava. Lung injury score, rib fracture number and disruption of the great vessels (aorta and vena cava) and other notable injuries (e.g. myocardial contusion) were the secondary safety outcomes.
Normality was investigated for each variable with the Shapiro-Wilke test, and non-parametric tests were applied where appropriate. Baseline characteristics were summarized and compared between groups to evaluate uniform between-group initial conditions. ROSC was compared between groups via Chi Squared test. Physiologic measures including CAP, CPP, and CBF were averaged in 1-minute epochs at baseline, during CPR, and after ROSC. The down-sampled measures were compared during the resuscitation phase using multivariable generalized estimating equations (GEE) accounting for between and within-subjects error and adjusting for time point, and group. Injury scores on the paired score sheets for each animal were compared by an adjudicator (JJM). Discrepancies were adjudicated in favor of the veterinarian member of the reviewer pair. Lung injury scores and rib fracture counts were compared between groups with the Wilcoxon Rank Sum test. Safety data were analyzed with the Bayesian Beta Binomial to determine if within-group toxicity exceeded an unacceptable level (30%) with a preselected posterior predictive threshold of 0.75(ptox).11 12 All other injuries were compared between groups with the Chi Squared test. All statistical analyses were performed in Stata 15 (Stata Corp. College Station, TX) with a threshold alpha level of 0.05.
Results
A total of 38 animals were included for primary hemodynamic analyses (1.5 inch n = 23; 1/3 APd n = 15), with 2 excluded for instrumentation errors (1 from each group), and reflected early termination of the 1/3 APd arm. Baseline physiologic measures, blood gases and electrolytes were similar between groups (Table 1). Of note, the mean overall APd was 7.6 (SD=0.2) inches, meaning the mean depth of chest compressions in the 1/3 APd group was 2.5 inches. Of 14 animals that received lateral x-ray imaging of the thorax, the ratio of compressible space to APd averaged 0.55 (SD: 0.03). After occlusion of the endotracheal tube, the majority (n = 34, 89.5%) of animals demonstrated a distinct bimodal hemodynamic deterioration trajectory (Figure 2), characterized by an initial rapid hypotensive episode followed by a rapid hypertensive rebound, culminating in loss of pulses. Four (11.5%) animals displayed a unimodal hemodynamic deterioration trajectory. Pulselessness was achieved overall in a mean of 7.2 minutes (SD: 0.9, min: 5.3, max: 8.9). This did not differ significantly between the 2 groups (1.5 inch : 7.3 [SD: 0.9] ; 1/3APd : 7.1 [SD: 1]; p = 0.587).
Table 1. Baseline Characteristics –
Animal characteristics at baseline are shown stratified by group with associated group comparison p-value parameters. All characteristics unless noted were determined to be normally distributed. Continuous variables represented as mean (sd) and were compared via t-test. Dichotomos variables represented as frequency (percentage) and compared via Chi Squared test.
| 1.5 inch n= 23 |
1/3 APd n = 15 |
Overall N = 38 |
p-value | |
|---|---|---|---|---|
| Male | 9 (39.1%) | 9 (60.0%) | 18 (47.4%) | 0.208 |
| Weight, kg | 26.0 (1.5) | 25.7 (1.4) | 25.9 (1.5) | 0.594 |
| APd, inches | 7.6 (0.2) | 7.6 (0.2) | 7.6 (0.2) | 0.443 |
| A-Time, min | 45.1 (15.2) | 46.6 (11.2) | 45.7 (13.6) | 0.729 |
| Heart Rate, BPM | 82 (15) | 93 (27) | 87 (21) | 0.117 |
| CBF, mL/Min | 107 (27) | 103 (29) | 106 (27) | 0.643 |
| CAP, mmHg | 97 (13) | 87 (15) | 92 (14) | 0.039 |
| CVP, mmHg | 6 (3) | 4 (2) | 5 (3) | 0.078 |
| pO2, mmHg | 83.1 (11.3) | 80.5 (10.0) | 82.1 (10.7) | 0.526 |
| pCO2, mmHg | 39.3 (3.8) | 37.5 (2.9) | 38.5 (3.5) | 0.132 |
| ETCO2, mmHg | 39 (3) | 36 (6) | 38 (4) | 0.053 |
Abbreviations: APd – Anterior-Posterior Diameter; A-Time – Anesthesia Time; BPM – Beats Per Minute; CAP – Central Arterial Pressure; CVP – Central Venous Pressure; CBF – Carotid Blood Flow; ETCO2 – End-Tidal Carbon Dioxide
Figure 2. Hemodynamic Crash Period –
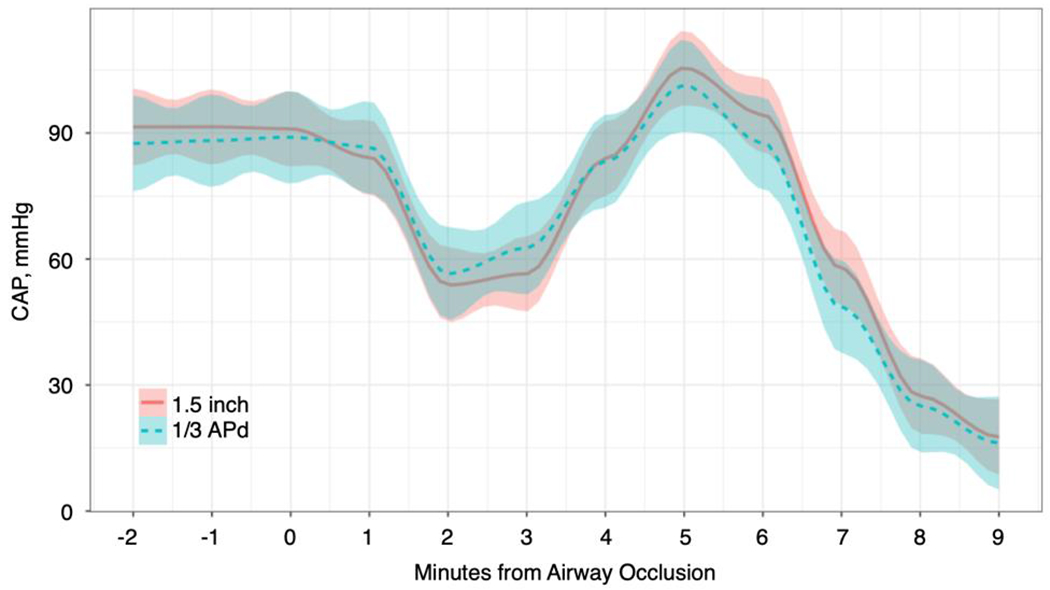
Central arterial pressure is shown in a smoothed range plot (mean with SD bounds) from airway occlusion to start of CPR.
Abbreviations: APd – Anterior-Posterior Diameter; CAP – Central Arterial Pressure
Heart rhythm at the initiation of CPR was most frequently ventricular fibrillation (79.0%), followed by pulseless electrical activity (21.1%), and did not differ significantly between groups. ROSC was achieved in a total of 9 (23.7%) animals and did not differ between groups (1.5 inch : 26.1% [n: 6] ; 1/3 APd : 20.0%[n: 3] ; p = 0.666). Only 6.7% (n = 2) of animals with an immediate pre-CPR rhythm of VF achieved ROSC, compared to 87.5% of those with PEA (p<0.001).
Injury characteristics are shown in Table 2. Both groups exhibited substantial CPR-related injury as evidenced by a high proportion with any fractured ribs, myocardial contusion and moderately high lung injury scores. However, significant group differences were observed in several injury categories. Number of rib fractures, any blood in the endotracheal tube and total lung injury score were significantly higher in the 1/3 APd group. Moreover, sternal fracture, hemothorax and aortic dissection were only observed in the 1/3 APd group. Toxicity occurred in zero animals in the 1.5 inch group (ptox=0.0001), and 7 animals in the 1/3 APd group (ptox=0.8180). The posterior probability threshold was exceeded in group 2 which warranted termination of the treatment arm for safety (see Figure 3). For this reason, enrollment in the 1/3 APd group was terminated early.
Table 2. Injury Characteristics –
Injuries sustained (including aggregate lung injury score) during resuscitation are summarized and stratified by group with associated group comparison p-value parameters.
| 1.5 inch n= 23 |
1/3 APd n = 15 |
Overall N = 38 |
p-value | |
|---|---|---|---|---|
|
| ||||
| Any Rib Fracture | 18 (78.3%) | 15 (100.0%) | 33 (86.8%) | 0.053 |
|
| ||||
| Number of Rib Fractures | 2.7 (1.7) | 6.7 (2.6) | 4.3 (2.9) | < 0.001 |
|
| ||||
| Any Blood in ETT | 0 (0.0%) | 12 (80.0%) | 12 (31.6%) | < 0.001 |
|
| ||||
| Any Sternal Fracture | 0 (0.0%) | 7 (46.7%) | 7 (18.4%) | < 0.001 |
|
| ||||
| Any Hemothorax | 0 (0.0%) | 6 (40.0%) | 6 (15.8%) | < 0.001 |
|
| ||||
| Any Aortic Dissection | 0 (0%) | 2 (13.3%) | 2 (5.3%) | 0.072 |
|
| ||||
| Any Vena Caval Dissection | 0 (0%) | 1 (6.7%) | 1 (2.6%) | 0.21 |
|
| ||||
| Any Myocardial Contusion | 15 (65.2%) | 10 (66.7%) | 25 (65.8%) | 0.703 |
|
| ||||
| Any Liver/Spleen Contusion | 1 (4.3%) | 2 (13.3%) | 3 (7.9%) | 0.315 |
|
| ||||
| Adjudicated LIS | ||||
| Median | 5 | 8.7 | 6.5 | < 0.001 |
| IQR | 5-5 | 8-10 | 5-8 | |
| Range | 1-7 | 6-10 | 1-10 | |
Abbreviations: APd – Anterior-Posterior Diameter; ETT – Endotracheal Tube, LIS – Lung Injury Score
Figure 3. Toxity Estimation by Group –
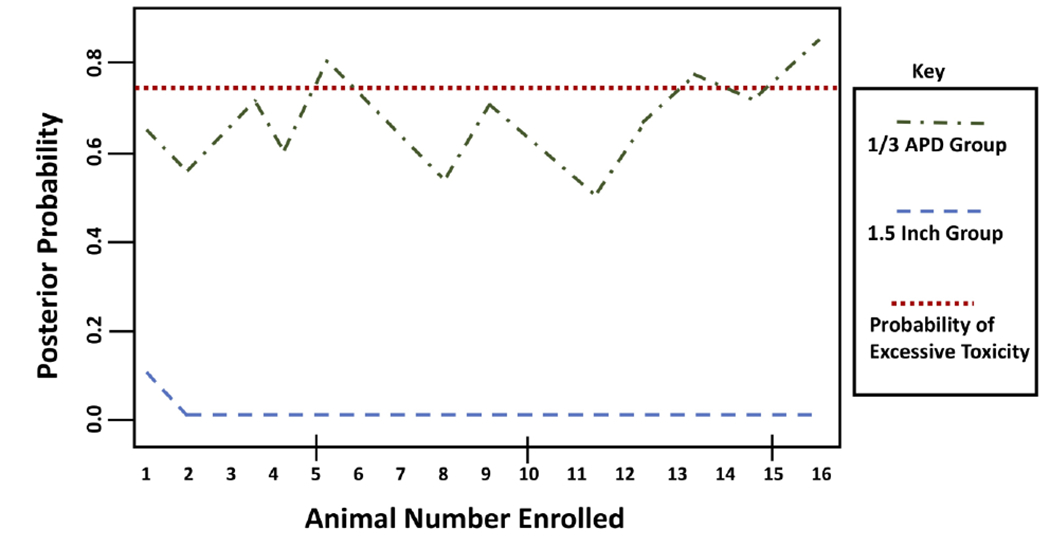
Bayesian derivation of a probability of excess toxicity (severe, unrecoveragble injury) tracked over the study period by group.
Abbreviations: APd – Anterior-Posterior Diameter
The development of physiologic measures during CPR is shown in Figure 4. Hemodynamic measures among animals with primary arrest rhythm VF changed over time (CAP, Coef: −1.52, p<0.001; CPP, Coef: −1.38, p<0.001; CBF, Coef: −2.70, p<0.001), however, measures did not differ significantly between treatment groups. Among animals with a primary arrest rhythm of PEA, meaningful assessment of the innate hemodynamics generated by CPR was difficult due to the simultaneous contribution of native cardiac output to blood flow and pressure during on-going compressions, as well as low sample size within this subgroup, as shown in the figure.
Figure 4. Hemodynamics During CPR Until First Pulse Check –
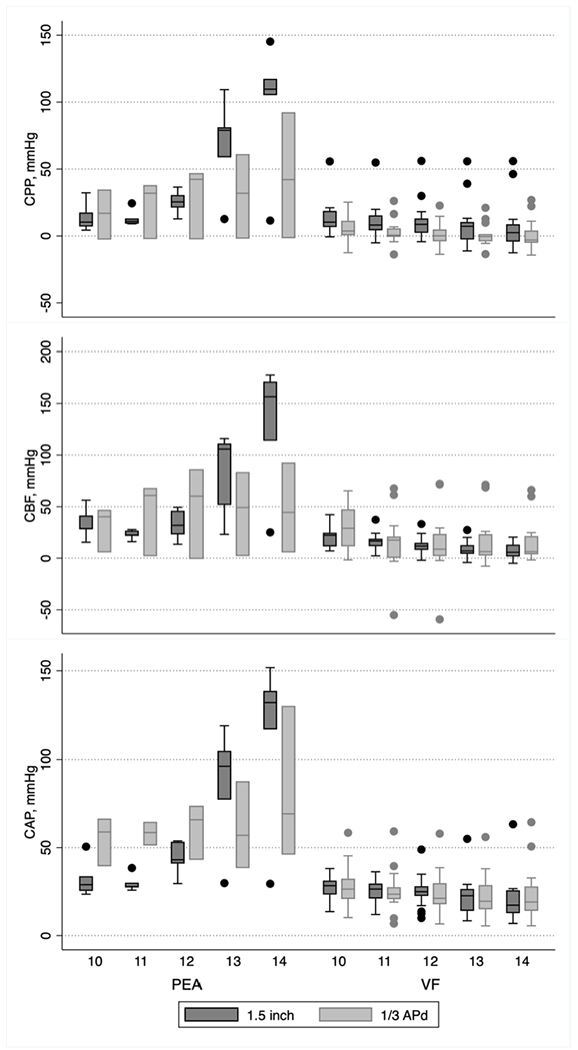
Box plots are shown during CPR until the first defibrillation / pulsecheck, stratified by PEA / VF rhythm status and treatment group for CPP, CBF and CAP. The timeline ranges from 10 to 14 minutes for each stratum, diving the left and right sides of each panel.
Abbreviations: APd – Anterior-Posterior Diameter; CAP – Central Arterial Pressure; CBF – Carotid Blood Flow; CPP – Coronary Perfusion Pressure
Discussion
We evaluated the safety and efficacy of the two primary recommended approaches to pediatric chest compression depth - 1/3 APd and 1.5 inches – on the basis of ROSC, hemodynamics, and injury in a pediatric swine model of asphyxia CA. Our principal findings establish that 1/3 APd chest compressions were excessively damaging without hemodynamic or ROSC benefit to justify the risk of serious injury in the context of this model. Sternal fracture, hemothorax, aortic dissection, vena caval dissection, airway blood, lung injury and total rib fractures were significantly higher or only present in 1/3 APd animals compared to animals receiving 1.5 inch compressions.
The mechanisms behind these injuries likely arise from the potential for excessively deep chest compressions to stress the anatomy, particularly the skeletal anatomy of the chest, at some or all phases of the resuscitation beyond innate safety limits. Figure 5 presents a radiograph showing the compression piston in the down position at 1/3 ADP and with it removed from the chest. A likely candidate for non-contusion soft tissue damage observed was weaponization of the fractured ribs, leading to lung lacerations and punctures during ongoing chest compressions and resultant hemothorax. Incidence of rib fracture in the contemporary resuscitation literature generally varies widely within and between clinical and preclinical studies, though among pediatric patients estimates tend to be low, perhaps reflecting malleability of younger anatomy.13–22
Figure 5. Thoracic Radiographs –
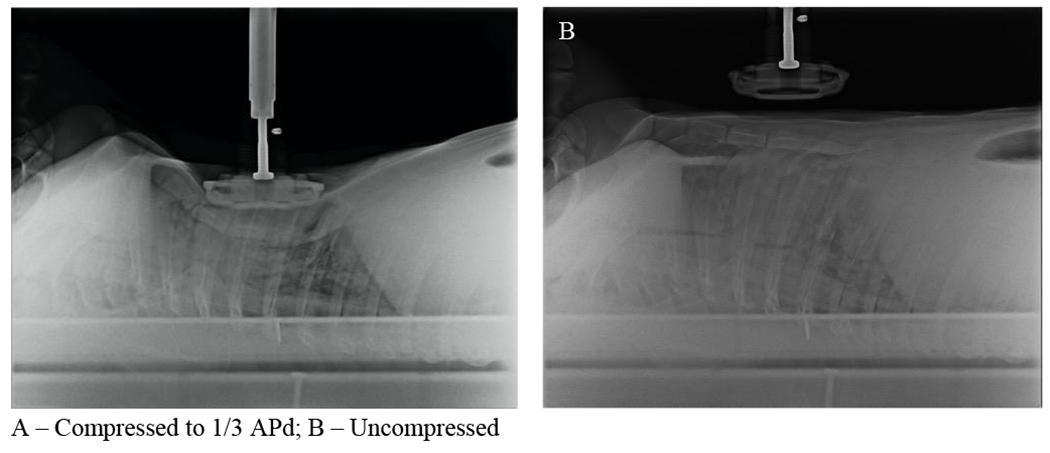
Lateral radiographs of the thorax of one supine pig are shown with (A) and without (B) the compressor piston deployed to 1/3 APd.
Details of the development, performance and sensitivity/specificity characteristics of the novel lung injury score utilized are beyond the scope of this study, but as described above, the individual lung injury score for the 1/3 APd group was nearly 4 points higher than the 1.5 inch group averaging nearly 9. For perspective, a score of 9 out of ten is not possible without significant damage to both lungs and all lobes therein, on average. This reinforces the notion that 1/3 APd approach was particularly deleterious for pulmonary injury. Previous granular characterization of lung injury due to chest compressions is limited, much like other areas of post-resuscitation anatomical injury characterization, and generally involves small sample sizes.8 14 16 18 21 23 24 Of studies that have investigated lung injury clinically, there is wide variability in estimated incidence and extremity of injury. Unfortunately, there is a paucity of studies in the literature specifically evaluating CPR-related pediatric lung injury, so recapitulation of our findings in an age-specific context is not possible. It follows, however, that lung lacerations observed in our study concurrently with rib fracture would likely correlate among clinical pediatric patients similarly owing to the simple homology between the mechanism in swine and humans.
Previous studies have elegantly explored pediatric chest compression depth limits using human computerized tomography (CT) imaging of human pediatric subjects.25 26 These studies describe the availability of compressible space in pediatric patients over the age range from infant to 17 years old as a function of the APd, indicating that 1/3 APd is well within the safe compressible depth range throughout the pediatric developmental period, as is 1.5 inches. The former finding clashes with our observation that 1/3 APd was highly damaging and not hemodynamically beneficial, although they are not necessarily mutually exclusive. Most such studies based in CT imaging do not specifically model compression-related damage, instead focusing on damage related to closure of the gap between the sternum and the spinal column. Xray imaging sampled irregularly in our study affords some basis for comparison between our study and those utilizing CT. Based on our measurements, it is a safe assumption that the 1/3 APd observed in our study corresponds to depths for which sufficient compressible space was available in the chest cavity to rule out the role of crushing trauma between sternum and spine. This is supported by our observed low rates of aortic and vena caval dissection.
Separately, our study found no significant difference in hemodynamic measures or ROSC between chest compression groups. Notably, compared to previous ALS studies in our laboratory, which have historically have been in a non-overdose non-asphyxial electrical VF model, hemodynamic output and rates of ROSC were extremely low in this study.27–29 In fact, epinephrine administered during the crucial first 5 minutes of resuscitation prior to the first defibrillation attempt yielded virtually no detectable impact on blood pressure or flow. This observation likely stems from the massive bolus of fentanyl, which is known to attenuate sympathetic tone and vasomotor response to catecholamines, administered concurrently with mechanical asphyxia in these animals.30 31 Under these conditions, coupled with profound hypoxia, hypercarbia and associated acidosis, it is likely that the epinephrine administered during resuscitation was minimally efficacious and that vascular tone was sufficiently compromised by fentanyl overdose to result in sub-optimal hemodynamics. The subsequent effects on ROSC were likely compounded by the delay to defibrillation during the initial BLS phase.
There are several limitations associated with this study. First, this is a preclinical study using a swine model of pediatric opioid-associated asphyxial cardiac arrest. Animal studies provide a plausible glimpse at associations between therapies and outcomes, and swine have long been established as the standard model species for examining cardiopulmonary resuscitation process effects, but our findings cannot be considered definitive until they are reinforced with detailed human clinical data. Moreover, the vast majority of asphyxial pediatric cardiac arrests are likely due to non-drug related causes32–35, limiting the generalizability of our hemodynamic and ROSC findings to a narrow slice of actual pediatric cardiac arrest. Second, as demonstrated by the differential between 1/3APd and 1.5 inch target depths in this study, our study model only includes animals of approximately 25kg and 4 months old. Fortunately, experiments are ongoing in our lab examining an earlier developmental stage, affording an opportunity to more fully understand the relative performance of these chest compression targets across the pediatric life cycle. Third, the study design we chose only provides limited insight into when in the course of resuscitation the observed injuries occurred. Some information can be gleaned from subanalyses of those with and without ROSC due to the different durations of sustained chest compressions, but this inference is limited by sample size. Fourth, we used a custom-built chest compression device. Being a unique device with unique properties, there is no certainty that our results will translate uniformly to other devices or human-performed CPR. Features that could matter include the device frame, drive, and piston head, all of which could influence injury or outcome. Because the range of variables in mechanical and human CPR performance is so great, we cannot generalize to all possible chest compression delivery approaches. What we can say is that the servo-driven device performed compressions at depths that were quite precise, and it is unlikely that human compressors could replicate that kind of precision. Fifth, as compressions wore on, remodeling of the chest occurred which could have affected the relative depth in both groups. Sixth, while the porcine chest anatomy is fairly similar to humans, there are differences that could have affected our results and the weights of the animals we used approximate the 50th percentile for seven year-olds and the 10th percentile for ten year-olds. Finally, while the defibrillation energy we chose for this study reflected the preponderance of experience from our laboratory on defibrillation in this size swine, it was higher than current pediatric clinical guidelines recommended. We believe erring on the side of laboratory optimum in this case was the best choice, though future studies may consider the implications of energy delivery on outcomes in this investigational context.
Conclusions
In a swine model of pediatric asphyxial out-of-hospital cardiac arrest, chest compressions delivered at a proportional depth of 1/3 Apd caused significantly more injury and did not yield any advantage on hemodynamic performance or ROSC. More work is needed to understand the translatability of these findings, which are most applicable to late childhood, to earlier stages of development.
Figure 6. Soft Tissue Injury Examples.
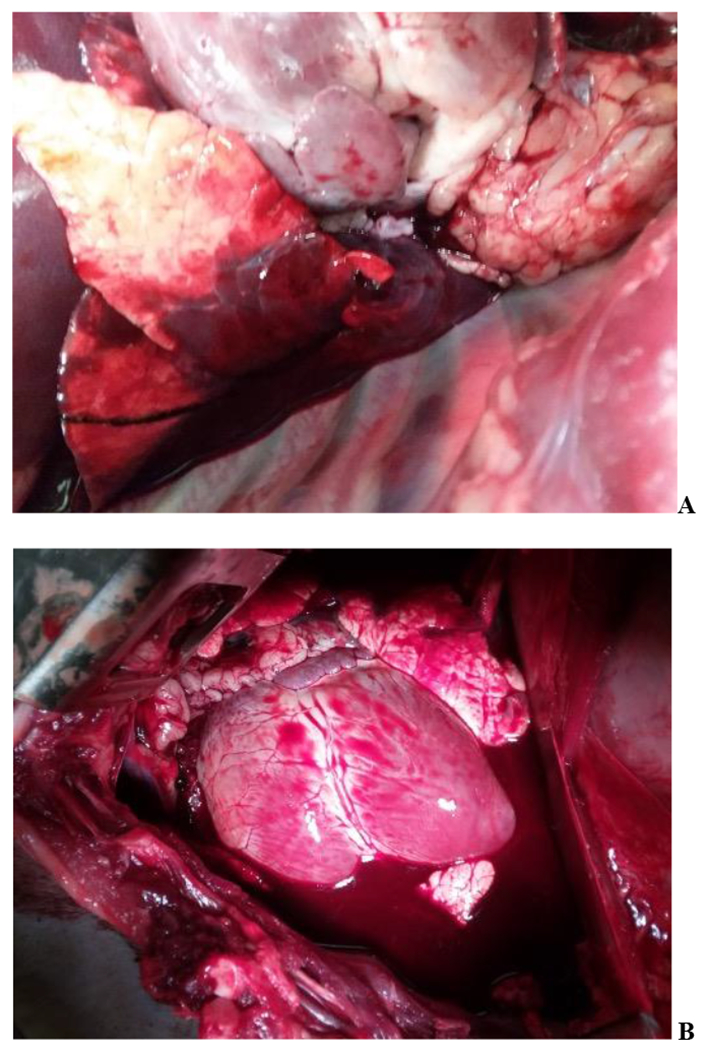
Findings at Necropsy: A – Lung Injury with Accompanying Myocardial Contusion; B – Diffuse Myocardial Contusion Accompanied by Hemothorax and Total Lung Injury Score of 20
Acknowledgements
We would like to acknowledge Dr. Jonathan Elmer, MD for his wisdom and assistance with the swine overdose model, Dr. Charity Galena Patterson, PhD for her statistical consultation, and the veterinary staff of the University of Pittsburgh Department of Laboratory Animal Resources for their generosity and hard work.
Funding Source:
Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Number R01HD095894 to Dr. Menegazzi. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr Genbrugge’s effort was supported by the Fulbright Visiting Scholars Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of Interest: Dr. Salcido performs fee-for-service work for ZOLL Medical. Drs. Salcido and Menegazzi hold a patent on a system for cardiopulmonary resuscitation.
References
- 1.Virani SS, Alonso A, Benjamin EJ, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020;141(9):e139–e596. doi: 10.1161/CIR.0000000000000757 [published Online First: 2020/01/30] [DOI] [PubMed] [Google Scholar]
- 2.Nichol G, Hallstrom AP, Ornato JP, et al. Potential cost-effectiveness of public access defibrillation in the United States. Circulation 1998;97(13):1315–20. doi: 10.1161/01.cir.97.13.1315 [published Online First: 1998/05/07] [DOI] [PubMed] [Google Scholar]
- 3.Andersen LW, Bivens MJ, Giberson T, et al. The relationship between age and outcome in out-of-hospital cardiac arrest patients. Resuscitation 2015;94:49–54. doi: 10.1016/j.resuscitation.2015.05.015 [published Online First: 2015/06/06] [DOI] [PubMed] [Google Scholar]
- 4.Fukuda T, Ohashi-Fukuda N, Matsubara T, et al. Trends in Outcomes for Out-of-Hospital Cardiac Arrest by Age in Japan: An Observational Study. Medicine (Baltimore) 2015;94(49):e2049. doi: 10.1097/MD.0000000000002049 [published Online First: 2015/12/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wissenberg M, Folke F, Hansen CM, et al. Survival After Out-of-Hospital Cardiac Arrest in Relation to Age and Early Identification of Patients With Minimal Chance of Long-Term Survival. Circulation 2015;131(18):1536–45. doi: 10.1161/CIRCULATIONAHA.114.013122 [published Online First: 2015/03/10] [DOI] [PubMed] [Google Scholar]
- 6.Panchal AR, Bartos JA, Cabanas JG, et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020;142(16_suppl_2):S366–S468. doi: 10.1161/CIR.0000000000000916 [published Online First: 2020/10/22] [DOI] [PubMed] [Google Scholar]
- 7.Topjian AA, Raymond TT, Atkins D, et al. Part 4: Pediatric Basic and Advanced Life Support 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Pediatrics 2021;147(Suppl 1) doi: 10.1542/peds.2020-038505D [published Online First: 2020/10/23] [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi S, Yamaguchi Y, Soejima K, et al. Incidence and characteristics of acute aortic dissection in patients with out-of-hospital cardiopulmonary arrest evaluated by non-contrast computed tomography. Eur Heart J Acute Cardiovasc Care 2020:2048872620923647. doi: 10.1177/2048872620923647 [published Online First: 2020/04/30] [DOI] [PubMed] [Google Scholar]
- 9.Zaidi HQ, Li S, Beiser DG, et al. The utility of computed tomography to evaluate thoracic complications after cardiopulmonary resuscitation. Resuscitation Plus 2020;3 doi: 10.1016/j.resplu.2020.100017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sundermann ML, Salcido DD, Koller AC, et al. Feasibility of Biosignal-guided Chest Compression During Cardiopulmonary Resuscitation: A Proof of Concept. Acad Emerg Med 2016;23(1):93–7. doi: 10.1111/acem.12844 [published Online First: 2016/01/01] [DOI] [PubMed] [Google Scholar]
- 11.Chen WF, Zhao NQ, Qin GY, et al. A Bayesian Group Sequential Approach to Safety Signal Detection. Journal of Biopharmaceutical Statistics 2013;23(1):213–30. doi: 10.1080/10543406.2013.736813 [DOI] [PubMed] [Google Scholar]
- 12.Wen S BG, Dey J. Bayesian Monitoring of Safety Signals in Blinded Clinical Trial Data. Ann Public Health Res 2015;2(2):1019. [Google Scholar]
- 13.Chen W, Weng Y, Wu X, et al. The effects of a newly developed miniaturized mechanical chest compressor on outcomes of cardiopulmonary resuscitation in a porcine model*. Crit Care Med 2012;40(11):3007–12. doi: 10.1097/CCM.0b013e31825d924d [published Online First: 2012/10/20] [DOI] [PubMed] [Google Scholar]
- 14.Dunham GM, Perez-Girbes A, Bolster F, et al. Use of whole body CT to detect patterns of CPR-related injuries after sudden cardiac arrest. Eur Radiol 2018;28(10):4122–27. doi: 10.1007/s00330-017-5117-0 [published Online First: 2017/11/11] [DOI] [PubMed] [Google Scholar]
- 15.Friberg N, Schmidbauer S, Walther C, et al. Skeletal and soft tissue injuries after manual and mechanical chest compressions. Eur Heart J Qual Care Clin Outcomes 2019;5(3):259–65. doi: 10.1093/ehjqcco/qcy062 [published Online First: 2019/01/17] [DOI] [PubMed] [Google Scholar]
- 16.Hoke RS, Chamberlain D. Skeletal chest injuries secondary to cardiopulmonary resuscitation. Resuscitation 2004;63(3):327–38. doi: 10.1016/j.resuscitation.2004.05.019 [published Online First: 2004/12/08] [DOI] [PubMed] [Google Scholar]
- 17.Ikeno F, Kaneda H, Hongo Y, et al. Augmentation of tissue perfusion by a novel compression device increases neurologically intact survival in a porcine model of prolonged cardiac arrest. Resuscitation 2006;68(1):109–18. doi: 10.1016/j.resuscitation.2005.05.024 [published Online First: 2005/12/06] [DOI] [PubMed] [Google Scholar]
- 18.Kim HI, Cha KC, Chung WJ, et al. Effect of chest compression on skeletal chest injuries: a retrospective study. Eur J Emerg Med 2020;27(1):59–63. doi: 10.1097/MEJ.0000000000000617 [published Online First: 2019/07/16] [DOI] [PubMed] [Google Scholar]
- 19.Liao Q, Sjoberg T, Paskevicius A, et al. Manual versus mechanical cardiopulmonary resuscitation. An experimental study in pigs. BMC Cardiovasc Disord 2010;10:53. doi: 10.1186/1471-2261-10-53 [published Online First: 2010/10/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez J, Fernandez SN, Gonzalez R, et al. Comparison between manual and mechanical chest compressions during resuscitation in a pediatric animal model of asphyxial cardiac arrest. PLoS One 2017;12(11):e0188846. doi: 10.1371/journal.pone.0188846 [published Online First: 2017/12/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smekal D, Johansson J, Huzevka T, et al. No difference in autopsy detected injuries in cardiac arrest patients treated with manual chest compressions compared with mechanical compressions with the LUCAS device--a pilot study. Resuscitation 2009;80(10):1104–7. doi: 10.1016/j.resuscitation.2009.06.010 [published Online First: 2009/07/15] [DOI] [PubMed] [Google Scholar]
- 22.Xanthos T, Pantazopoulos I, Roumelioti H, et al. A comparison of autopsy detected injuries in a porcine model of cardiac arrest treated with either manual or mechanical chest compressions. Eur J Emerg Med 2011;18(2):108–10. doi: 10.1097/MEJ.0b013e32833e79cf [published Online First: 2010/08/25] [DOI] [PubMed] [Google Scholar]
- 23.Mateos Rodriguez AA, Pascual JM, Vallejo FP, et al. Lung injuries secondary to mechanical chest compressions. Resuscitation 2012;83(10):e203. doi: 10.1016/j.resuscitation.2012.03.040 [published Online First: 2012/07/24] [DOI] [PubMed] [Google Scholar]
- 24.Miller AC, Rosati SF, Suffredini AF, et al. A systematic review and pooled analysis of CPR-associated cardiovascular and thoracic injuries. Resuscitation 2014;85(6):724–31. doi: 10.1016/j.resuscitation.2014.01.028 [published Online First: 2014/02/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braga MS, Dominguez TE, Pollock AN, et al. Estimation of optimal CPR chest compression depth in children by using computer tomography. Pediatrics 2009;124(1):e69–74. doi: 10.1542/peds.2009-0153 [published Online First: 2009/07/01] [DOI] [PubMed] [Google Scholar]
- 26.Jin SY, Oh SB, Kim YO. Estimation of optimal pediatric chest compression depth by using computed tomography. Clin Exp Emerg Med 2016;3(1):27–33. doi: 10.15441/ceem.16.119 [published Online First: 2016/10/19] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chonde M, Flickinger KL, Sundermann ML, et al. Intra-Arrest Administration of Cyclosporine and Methylprednisolone Does Not Reduce Postarrest Myocardial Dysfunction. Biomed Res Int 2019;2019:6539050. doi: 10.1155/2019/6539050 [published Online First: 2019/07/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds JC, Salcido DD, Menegazzi JJ. Coronary perfusion pressure and return of spontaneous circulation after prolonged cardiac arrest. Prehosp Emerg Care 2010;14(1):78–84. doi: 10.3109/10903120903349796 [published Online First: 2009/12/02] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds JC, Salcido DD, Menegazzi JJ. Conceptual models of coronary perfusion pressure and their relationship to defibrillation success in a porcine model of prolonged out-of-hospital cardiac arrest. Resuscitation 2012;83(7):900–6. doi: 10.1016/j.resuscitation.2012.01.007 [published Online First: 2012/01/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taneyama C, Goto H, Kohno N, et al. Effects of fentanyl, diazepam, and the combination of both on arterial baroreflex and sympathetic nerve activity in intact and baro-denervated dogs. Anesth Analg 1993;77(1):44–8. doi: 10.1213/00000539-199307000-00009 [published Online First: 1993/07/01] [DOI] [PubMed] [Google Scholar]
- 31.Torralva R, Janowsky A. Noradrenergic Mechanisms in Fentanyl-Mediated Rapid Death Explain Failure of Naloxone in the Opioid Crisis. J Pharmacol Exp Ther 2019;371(2):453–75. doi: 10.1124/jpet.119.258566 [published Online First: 2019/09/08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ackerman M, Atkins DL, Triedman JK. Sudden Cardiac Death in the Young. Circulation 2016;133(10):1006–26. doi: 10.1161/CIRCULATIONAHA.115.020254 [published Online First: 2016/03/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardai A, Berdowski J, van der Werf C, et al. Incidence, causes, and outcomes of out-of hospital cardiac arrest in children. A comprehensive, prospective, population-based study in the Netherlands. J Am Coll Cardiol 2011;57(18):1822–8. doi: 10.1016/j.jacc.2010.11.054 [published Online First: 2011/04/30] [DOI] [PubMed] [Google Scholar]
- 34.Jayaram N, McNally B, Tang F, et al. Survival After Out-of-Hospital Cardiac Arrest in Children. J Am Heart Assoc 2015;4(10):e002122. doi: 10.1161/JAHA.115.002122 [published Online First: 2015/10/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meert KL, Telford R, Holubkov R, et al. Pediatric Out-of-Hospital Cardiac Arrest Characteristics and Their Association With Survival and Neurobehavioral Outcome. Pediatr Crit Care Med 2016;17(12):e543–e50. doi: 10.1097/PCC.0000000000000969 [published Online First: 2016/09/30] [DOI] [PMC free article] [PubMed] [Google Scholar]


