Abstract
Cancer is considered a life-threatening disease, and several factors are involved in its development. Chemokines are small proteins that physiologically exert pivotal roles in lymphoid and non-lymphoid tissues. The imbalance or dysregulation of chemokines has contributed to the development of several diseases, especially cancer. CCL19 is one of the homeostatic chemokines that is abundantly expressed in the thymus and lymph nodes. This chemokine, which primarily regulates immune cell trafficking, is involved in cancer development. Through the induction of anti-tumor immune responses and inhibition of angiogenesis, CCL19 exerts tumor-suppressive functions. In contrast, CCL19 also acts as a tumor-supportive factor by inducing inflammation, cell growth, and metastasis. Moreover, CCL19 dysregulation in several cancers, including colorectal, breast, pancreatic, and lung cancers, has been considered a tumor biomarker for diagnosis and prognosis. Using CCL19-based therapeutic approaches has also been proposed to overcome cancer development. This review will shed more light on the multifarious function of CCL19 in cancer and elucidate its application in diagnosis, prognosis, and even therapy. It is expected that the study of CCL19 in cancer might be promising to broaden our knowledge of cancer development and might introduce novel approaches in cancer management.
Keywords: chemokine, cancer, CCL19, biomarker, immunotherapy
This study is focused on the role of CCL19 in cancer development. It has been shown that CCL19 exerts conflicting functions in the tumor microenvironment. Using CCL19-based therapies might introduce novel approaches in cancer treatment.
Graphical Abstract
Graphical Abstract.
Introduction
Inflammation is considered a vital component of the tumor microenvironment, and it is proposed as a hallmark of cancer [1]. Chemokines are a group of chemotactic cytokines that orchestrate the proper movement of immune cells through specific binding to corresponding receptors, thus contributing to the process of immune regulation and inflammatory response [2]. Therefore, it has been proposed that chemokines can modulate the recruitment of several cells in the tumor microenvironment [3, 4]. Based on the location of two N-terminal cysteines (C), these cytokines are divided into four groups, including CC, CXC, CX3C, and C chemokines. CC motif chemokine ligands (CCL) encompass large groups of chemokines and are involved in various homeostatic, physiological, and even pathological conditions. Among CC chemokines, CCL19 is almost the most well-known chemokine for its homeostatic and physiological functions in the development of primary and secondary lymphoid organs [5]. Besides various physiological conditions, CCL19 is also involved in pathological situations, e.g. cancers [6]. Therefore, this study aimed to comprehensively review several functions of CCL19 in cancer.
CCL19; characteristics, expression, the main function
For the first time, CCL19 was described in 1997 through bioinformatics by two independent studies. Although it was initially called macrophage proinflammatory human 3-β (MIP3-β) or EBI1-ligand chemokine (ELC), it was then renamed to CCL19 due to having 20–30% homologies to CC chemokines [7, 8]. Although CC chemokine genes are located on chromosome 17, the CCL19 gene is mapped on chromosome 9p13. CCL19 is highly expressed in lymphoid organs, including the thymus and lymph nodes. Therefore, it exerts physiological and homeostatic functions in developing the immune system. In addition, CCL19 has been confirmed to be moderately expressed in non-lymphoid organs, including the colon and trachea. Moreover, the small intestine, lung, spleen, kidney, and stomach express CCL19 at low levels [9]. Furthermore, peripheral blood leukocytes, fetal liver, and bone marrow were almost negative for CCL19 mRNA expression [8].
Proposed receptors for CCL19
CCR7 is the primary and functional receptor for CCL19, which is also considered a shared receptor for CCL21. CCR7, like other chemokine receptors, is a member of the G protein-coupled receptor (GPCR) family. This receptor is expressed in T and B cells and various lymphoid tissues, and it is physiologically involved in lymphocyte trafficking between lymph nodes, the spleen, and other tissues [10]. In addition to CCR7, other proteins have been shown to bind to CCL19. These so-called receptors (also known as decoy receptors) obstruct the CCL19/CCR7 interaction. CCX-CKR (also called CCR11 or CCRL1), as a member of the atypical chemokine receptor (ACKR) family, is capable of binding to CCL19, CCL21, and CCL25. However, this receptor cannot trigger the typical signaling cascades like other chemokine receptors. It has been shown that CCX-CKR mediates CCL19 internalization into the cell, leading to its degradation. Therefore, it is proposed that CCX-CKR scavenges CCL19 to modulate the consequences of CCL19/CCR7 interaction [11]. Chemokine receptor on activated macrophages (CRAM; also called CCRL2) is another member of ACKRs that can bind CCL19. Like CCX-CCR, CRAM also acts as a scavenger receptor. CRAM binding to CCL19 could not activate cell signaling, and it functions as a CCL19 recycling receptor that competed with CCR7 to modulate CCR7/CCL19 signaling [12].
As mentioned, chemokines have many functions, but they are best known for their crucial role in coordinating cell migration during homeostasis and inflammation. However, they play a role in organogenesis as well as tumor cell growth and spreading [13, 14]. The chemokine receptor CCR7 and its ligands (CCL19 and CCL21) play an important role in regulating the trafficking of immune cells, including lymphocytes and DCs, in secondary lymphoid tissues. In addition, CCL21, along with CCL19, promotes the proper navigation and compartmentation of immune cells, including T cells and DC, in secondary lymphoid tissues [15]. CCL19 and CCL21 are expressed in lymph nodes, although their expression patterns are distinct from one another [15]. CCL19 is only produced and presented in the T-cell zone in human lymph nodes. In contrast, CCL21 is produced in the T-cell zone and is transcytosed to high endothelial venules (HEV) [16]. CCL19 and CCL21 are also expressed by reticular cells inside the T-cell zone, in which CCL21-expressing cells are more numerous than CCL19-expressing cells in the T-cell zone’s periphery. This suggests that the T-cell zone’s CCL21-producing tissue is greater than the T-cell zone’s CCL19-producing tissue [15].
In various T-cell zones’ sub-regions, the profiles of the overlapping CCL19 and CCL21 fields may differ. There is also a considerable discrepancy in the amounts of CCL19 and CCL21 production in secondary lymphoid tissues, as CCL21 production is up to 100-fold greater than CCL19 production; however, the precise difference is not established in sub-regions [16]. CCL19 exclusively shows soluble patterns in secondary lymphoid tissues, whereas CCL21 is observed in soluble and insoluble forms. According to the investigations, both CCL19 and CCL21 have a similar binding affinity for the CCR7 receptor and have similar effects on calcium immobilization and G-protein activation at the cellular level. CCL19, but not CCL21, is the sole chemokine that effectively desensitizes and internalizes CCR7 [15]. Despite the fact that both CCL19 and CCL21 are potent chemoattractants for T cells, as demonstrated by in vitro chemotaxis tests, their unique functions in controlling T-cell trafficking in secondary lymphoid tissues are yet unknown. It has been demonstrated that CCL21, but not CCL19, is necessary for the recruitment of T cells and DCs to secondary lymphoid organs employing CCL21 and CCL19 deficient mice. This observation, together with the fact that CCL19 synthesis is much lower in secondary lymphoid organs, has made understanding the role of CCL19 in DC and lymphocyte trafficking in these organs even more challenging [15].
Physiological Functions of CCL19
CCL19 is produced by stromal cells of lymph nodes, mature dendritic cells, the spleen, and HEV [17]. CCL19’s interactions with its receptor (CCR7) orchestrate lymphoid organ organization, immunological response initiation, and immune tolerance induction [18]. CCL19 can initiate inside-out signaling to several integrins, including αLβ2, α4β1, α4β7 on lymphocytes. In lymph nodes, soluble chemokines like CCL19 may activate high-affinity integrin conformations, although it takes 10–30 min [19]. Chemokines promote the survival of T cells as CCR7 signaling protects CD8+ T cells from apoptosis. CCL19 plays a critical role in naïve T-cell homeostasis through its anti-apoptotic activity [5]. CCL19 also promotes the endocytosis capacity of DCs, co-stimulatory molecule expression, production of IL-12, TNFα, and IL-1β, and decreases DC apoptosis, hence indirectly increasing T-cell proliferation and Th1 polarization [20]. CCL19 can also serve as a survival signal by activating the PI3K/GSK3/NF-κB pathway. The pro-apoptotic transcription factor FoxO1 is phosphorylated and inhibited by CCR7-mediated Akt activation. Moreover, CCL19 is essential for the maturation of TLR-activated DCs [20]. It has been demonstrated that CCL19 can increase the proliferation of natural killer (NK) cells triggered by IL-2 [20, 21].
CCL19 is a chemokine that regulates the recruitment of CCR7-expressing T-cells as well as the homeostatic trafficking of lymphocytes and dendritic cells along a chemokine gradient. It also plays a role in the enhancement of T-cell responses and antitumor immunity [22]. T-cell responses are influenced by CCL19, which is the only chemokine known to promote CCR7 phosphorylation and internalization through the β-arrestin pathway efficiently. This leads to receptor desensitization and DC (as antigen-presenting cells) movement, which in turn affects T-cell responses [22, 23]. CCL19 internalization in T cells is clearly concentration- and time-dependent, which differs from CCL21 as another ligand for CCR7 [24]. Compared to other chemokines, CCL19 has been found to be much more powerful in activating G-protein signaling, eliciting chemotaxis, and increasing Ca2+ [25]. The signal induced by CCL19 is robust and short-lived; thus, CCL19 promotes efficient signaling of CCR7 via Gαi, which allows for subsequent β-arrestin employment and internalization of the receptor. Moreover, it has been shown that CCL19 is 10–100 times more powerful in inducing the directed migration of DCs than CCL21, indicating that these two chemokines have distinct effects on DC chemotaxis responses [24, 26].
Interestingly, it was discovered that the CCL19−/− mice exhibited a phenotype that was comparable to that of the CCR7−/− mouse. Conversely, when comparing the behavior of wild-type TCD4+ cells transferred into the CCL19−/− strain with that of the respective wild-type strain, it was found that they were more slowly cleared from lymph nodes. In a similar vein, mice treated with the ELC8–83 (CCL19 antagonist) had a substantial increase in the lymph nodes’ T-cells population when compared to mice treated with vehicle alone. Both of these investigations found that a decrease in CCR7 signaling was associated with T lymphocyte persistence in the peripheral lymph nodes [27]. T cells leave the lymph nodes via sphingosine-1-phosphate receptor 1 (S1P1) and lose their capacity to move to CCL21 by down-regulating CCR7 as a result of this mechanism. Studies have shown that CCR7 internalizes more efficiently in response to CCL19 on active DCs than CCL21, which attracts T lymphocytes to lymph nodes. The mean surface level of S1P1 expression is lower in CCR7−/− T-cells than in the wild-type, suggesting that CCR7 is involved in S1P1 up-regulation [27].
CCL19 and cancer
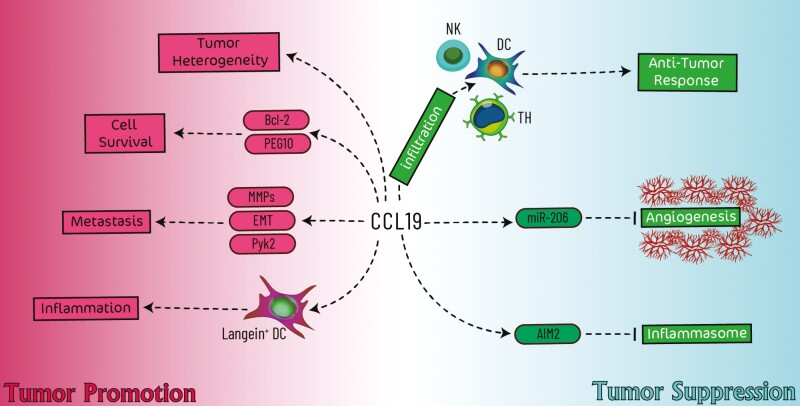
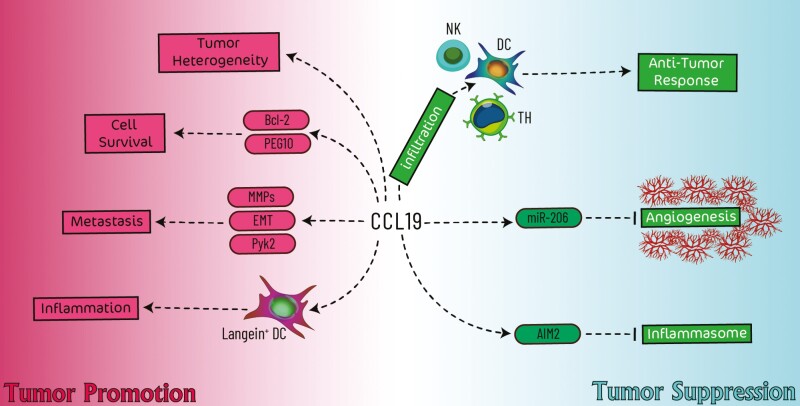
The procedure of cancer development and progression has not yet been fully elucidated, and the contribution of several aspects has been attributed to the initiation of cancer development. Among several proposed factors, inflammation has been considered one of the initiators [34]. Upon cancer progression, inflammatory cells such as recruited immune cells and activated myofibroblasts are infiltrated into the tumor nest by several mediators, including cytokines, chemokines, and hypoxia [35]. Among secreted factors, chemokines modulate inflammation in the tumor microenvironment by recruiting several inflammatory cells. Previous studies have indicated that chemokines, including CXCL14 and CXCL16, are vital components of the tumor microenvironment [3, 4]. In the following, we overviewed the importance of CCL19’s role in tumor development (Fig. 1).
Fig. 1.
Schematic illustration of CCL19’s involvement in cancer development. The tumor-suppressive role of CCL19 (shown in the blue area) relies on the enhancement of anti-tumor responses and inhibition of angiogenesis. In contrast, the tumor-promoting role of CCL19 (shown in the red area) is attributed to the inhibition of cell apoptosis, promotion of metastasis and tumor heterogeneity, and inflammation.
CCL19-induced signaling pathways in tumor cells
CCR7 is a GPCR that, upon ligation to CCL19, triggers signal transduction. It has been shown that CCL19-induced signaling via CCR7 differs between tumor and normal cells [28]. It is also mentioned that CCL19-induced signaling overlaps CCL21-induced signaling via CCR7, indicating that CCL21 might be involved in the pathogenesis of tumors in the absence of CCL19 [28]. CCL19 ligation to CCR7 induces phosphorylation of this receptor on four possible amino acid residues, mainly by protein kinase C (PKC) enzymes [29]. Treatment of PCI-37B cells with CCL19 induced protein kinase Cα (PKCα) without its upregulation. Upon PKCα activation, NF-κB was induced in these cells and was translocated into the nucleus. Hence, CCL19 induces PKCα/NF-κB in PCI-37B cells in a CCR7-dependent manner [30]. Moreover, incubation of PCI-37B cells with CCL19 activates PI3K signaling, which consequently induces Cdc42 in these cells to mediate cell migration [31]. mTOR and p70S6K are other PI3K downstream kinases that are phosphorylated and activated upon CCL19/CCR7 interaction in PCI-37B and PCI-4B cells. This pathway stimulates cell transition from the G1/S phase, thereby promoting the cell cycle [32]. In B-CLL, CCL19 ligation to CCR7 has led to the activation of PI3K/Akt, Rho-ROCK/MLC, and ERK1-2/p38-SAPK pathway, which consequently contributes to actin-myosin contraction, leading to invasion and cell migration [33]. Richardson et al. have shown that expression of a kinase called Zeta-chain-associated protein kinase 70 (ZAP-70) in B-CLL increases cell responsiveness to CCL19 and leads to F-actin polymerization and cellular migration, indicating the importance of this signaling protein in CCL19-dependent tumor cell migration [34]. In conclusion, CCL19-induced signaling encompasses several pathways, eventually leading to tumor development.
Dysregulation of CCL19 in cancer
As mentioned before, CCL19 is expressed at high levels in many lymphoid organs. Therefore, its expression at high levels in these organs supports its homeostatic and physiologic functions. However, CCL19 dysregulation in several pathologic conditions such as cancer has attracted much attention, as in some cases, its expression is elevated while it is downregulated in others. Hence, the CCL19 expression pattern is proposed to be context-dependent, and several factors might be involved in the up- or down-regulation of this chemokine in the tumor microenvironment.
Initiation and triggering of signaling pathways have been considered as one of the regulators of gene expression. It has been found that the epithelial signal transducer and activator of transcription 3 (STAT3) regulate tumor progression through modulating leukocytic recruitment into the tumor microenvironment. Activation of STAT3 signaling controls T-cell trafficking through inducing or inhibiting chemokine expression. STAT3 loss in the epithelium mediates several events that eventually promote the expression of CCL19 in the tumor microenvironment, indicating the importance of STAT3 in regulating CCL19 expression in the inflamed colon [35].
Human endogenous retroviruses (HERVs) are a group of genes that are derived from the ancestral integration of exogenous retroviruses, and their expression is linked to several diseases, especially cancers. These genes exert immunomodulatory functions through the modulation of immune-related genes. It has been shown that HREV-H expression in tumor cells is required for CCL19 production. An HERV-H-derived peptide called H17 induces the Twist/PI3K pathway in tumor cells and stimulates CCL19 production in human pancreatic cancer MIAPaca cells [36].
A group of transcription factors called runt-related transcription factors (RUNXs) has pivotal roles in the normal or neoplastic development of cells. RUNX3 acts as a tumor suppressor in most cancers, especially NSCLC. Aberrant methylation of the RUNX3 gene promoter occurs during tumorigenesis and is more common in invasive compared with pre-invasive lung adenocarcinoma lesions. Loss of RUNX3 in A549 cells was correlated to the dysregulation of a set of chemokines, particularly CCL19. RUNX3 knockdown in NSCLC cells decreased CCL19 expression, which was associated with tumor cell metastasis into the bone, indicating that RUNX3 is a CCL19 gene regulator [37].
Tumor-suppressive roles of CCL19
One of the important mechanisms of chemokines in tumor inhibition is attributed to the activation of anti-tumor immune responses. Since CCL19 is expressed in secondary lymphoid tissues and is involved in homing of T cells and DCs, it is assumed that its expression might contribute to tumor regression. In addition to immune stimulation, CCL19 also exerts other anti-tumor functions, which are described in the following.
CCL19 activates anti-tumor immune responses
Inflammation acts as a double-edged sword in tumor development. Acute inflammation triggers an antitumor immune response that assists in cancer cell killing, whereas cancer-induced chronic inflammation promotes tumor cell growth and resistance to therapeutic agents [38]. Therefore, many studies have shown that inflammation is intimately associated with cancer development and progression, as well as the effectiveness of anti-cancer therapy [39]. Chronic inflammation contributes to immunosuppression, resulting in a favorable microenvironment for tumorigenesis, progression, and metastasis [40]. Malignancy and its development have both been linked to chronic inflammation in most cancers. In contrast, it has been observed that acute inflammation caused by exogenous stimulators can increase antitumor immunity by enhancing the maturation and activity of DCs and the induction of effector T-cells [38].
As mentioned earlier, CCL19 infiltrates immune cells, including helper T-cells and antigen-presenting cells (APCs), into the tumor microenvironment and consequently exerts immune surveillance. In a murine model of lung cancer, intra-tumoral administration of recombinant CCL19 exhibited an increased infiltration and frequency of CD4+ and CD8+ T-cells as well as DCs at the tumor sites, which was accompanied by elevated levels of IFN-γ, CXCL9/10, IL-12, and GM-CSF but a decrease in the immunosuppressive factors PGE2 and TGFβ. Transferring activated T-cells from CCL19-treated mice to naïve mice endowed the anti-tumor efficacy of CCL19 therapy to the recipient mice [41]. Similar results were observed in the murine model of colorectal cancer (CRC). The administration of recombinant mouse CCL19 to the tumor site enhanced levels of IFN-γ and IL-12 in the tumor microenvironment and plasma and consequently suppressed CRC tumorigenesis and prolonged overall survival of mice by inducing anti-tumor immune responses [42]. Moreover, the introduction of CCL19 to breast tumor-bearing mice induced NK cells and inhibited tumor growth [43]. These data confirmed the immune-stimulatory and anti-tumor functions of CCL19.
Besides specific anti-tumor immune responses, CCL19 function against tumor cells relies on other immune system components such as inflammasomes. Absent in melanoma 2 (AIM2), as a cytosolic double-stranded (dsDNA) sensor, is considered a crucial inflammasome component that activates immune responses, inflammation, and even cell death in a caspase-1-dependent manner. Activation of AIM2 has been shown to be involved in tumor suppression, tumor cell growth inhibition, and cell cycle arrest. Since expression of AIM2 and CCR7/CCL19 have been downregulated in progressive gastric cancer compared with an early stage of gastric cancer, it is proposed that these genes might be involved in the initiation of tumor development. Administration of CCL19 to AIM2-transfected gastric cancer cells (AGS and MGC-803 cells) enhanced AIM2 expression, indicating an association between CCL19 and AIM2. After transfection with AIM2, gastric cancer cells showed higher expression of IL-1β and IL-18 and promoted LDH release, which were further elevated upon CCL19 administration. In addition, pro-apoptotic proteins including BID and caspase-3 were also increased in this experiment. Furthermore, CCL19 inhibited GC cell invasion and migration, which was reversed by CCR7 siRNA, indicating the AIM2-dependent anti-tumorigenic activity of CCL19 [44].
CCL19 and its anti-angiogenic properties
Angiogenesis is considered a vital step in tumor development and metastasis via the formation of new micro-vessels in the tumor microenvironment. This process is closely regulated by angiogenic factors, including VEGFA and hypoxia-inducible factor (HIF)-1α. In a study by Xu et al., it has been shown that the administration of CCL19 to colorectal cancer cells showed anti-angiogenic activities, which mechanistically rely on miRNA dysregulation. In this study, it was found that treatment of colorectal cancer cells (SW1116 and SW620 cells) with CCL19 significantly induced miR-206 in these cells. Overexpression of miR-206, which is proposed as a tumor-suppressor miRNA, consequently suppressed expression of VEGFA in these cells via inhibiting the MET/ERK/Elk-1/HIF-1α pathway. Down-regulation of VEGFA expression via CCL19 administration reduced the proliferation, migration, and tubule formation ability of HUVEC cells. In addition to in vitro observations, treatment of colon carcinoma-bearing murine models with CCL19 decreased micro-vessels, indicating that CCL19 could reduce angiogenesis, tumor size, and weight of tumor nodules in vivo [45].
Tumor-promoting roles of CCL19
In addition to the tumor-suppressive roles, CCL19 binding to CCR7 on tumor cells might initiate tumor development.
CCL19 and its role in tumor heterogeneity
Heterogeneity of tumor cells is considered a hallmark of cancer. This phenomenon mainly results from the imperfection of DNA replication and mutations, leading to a diverse population of cancer cells that can show different and distinct phenotypic and morphologic profiles, including metabolism, gene expression, proliferation, etc. The heterogeneity of tumor cells creates major challenges and influences the effectiveness of the treatments [46]. Therefore, during routine treatment of heterogenic tumors, some tumor cells are not killed and introduce drug resistance. Moreover, due to heterogeneity and genetic differences, a prognostic and diagnostic biomarker may be hard to define [47]. It has been shown that CCL19 exposure and its increased gradients enhanced breast tumor cell heterogeneity and altered tumor cell chemokinesis. Therefore, these results have highlighted the importance of cytokine and chemokine gradients in the tumor microenvironment due to inducing heterogeneity of cancer cells [48].
Anti-apoptotic and pro-survival functions of CCL19
Tumor cells are mainly resistant to common therapies such as chemotherapies that are considered potent inducers of apoptosis. These cells block apoptosis through down-regulation of pro-apoptotic pathways and upregulation of anti-apoptotic signals or inducing faulty apoptotic signaling [49]. As mentioned in the signaling section, CCL19 ligation to CCR7 on HNSCC cells constitutively increased phosphorylation of Akt, independent of epidermal growth factor receptor (EGFR) signaling, and upregulated expression of anti-apoptotic Bcl-2 protein. Up-regulation of Bcl-2 in these cells has led to inhibition of cisplatin-induced apoptosis, indicating the importance of CCL19/CCR7 signaling in cisplatin resistance [50].
Another mechanism involved in CCL19-mediated tumor development relies on the paternally expressed gene 10 (PEG10) gene. This gene is associated with a mediator of apoptosis called SIAH1. Exogenous expression of PEG10 endows oncogenic activity and transfection of hepatoma tumor cells (HepG2 cells) with PEG10 antisense results in suppression of cancer cell growth [51]. CD23+CD5+ B-ALL cells have been shown to express higher levels of CCR7 and CXCR5 than CD19+ B cells from normal peripheral blood. CCR7+ and CXCR5+ B-ALL cells activated protein kinase C (PKC) in response to CCL19 and CXCL13 and selectively increased PEG10 expression, which consequently did not lead to up-regulation of anti-apoptotic proteins but stabilized caspase-3 and -8, indicating that CCL19 and CXCL13 synergically rescued B-ALL cells from TNFα-mediated apoptosis in a PEG10-dependent manner [52]. In addition to the induction of resistance to apoptosis, CCL19/CXCL13-activated CD23+CD5+ B-ALL cells also PEG10-dependently upregulated IL-10 production. IL-10 secretion from these malignant cells impaired the cytotoxicity of CD8+ T-cells (CTLs), allowing CD23+CD5+ B-ALL cells to escape from host immune surveillance. It is worth noting that when exposed to CXCL13 and CCL19, normal CD19+ B cells and CD23+CD5 B cells from cord blood did not exhibit anti-apoptotic or immune-regulatory responses [53].
The pro-metastatic activity of CCL19
Tumor metastasis is a process that relies on several factors. Infiltration and invasion of lymphoma cells into the CNS and brain and retention of these cells are major complications in lymphomas, which exacerbate the disease and is considered a challenge for treatment. Non-specific reactions such as damage, gliosis, and aging might enhance the trafficking of lymphoma cells into the CNS and elevate the risk of CNS lymphoma. In gliosis, it has been shown that astrocytes produce CCL19 considerably, and the interaction of CCR7 on lymphoma cells with astrocyte-produced CCL19 increases infiltration of these cells into the CNS in mice. Clinical evidence for this observation was confirmed by showing that CCL19 expression was elevated in the cerebrospinal fluid (CSF) of patients with primary and secondary central nervous system lymphoma (PCNSL and SCNSL) [54]. In addition to the attraction of lymphoma cells into the CNS, it has also been demonstrated that CCL19 expression in mouse brains is also required for the retention of lymphoma cells. Collectively, astrocyte-derived CCL19 is proposed to be the crucial factor for CNS lymphoma and might be considered a prognostic factor for spreading lymphoma into the CNS [54]. Buonamici et al. also confirmed the infiltration of T-ALL cells into the CNS in a CCL19/CCR7-dependent manner [55].
The expression of extracellular matrix (ECM) modifying enzymes such as matrix metalloproteinases (MMPs) is considered a crucial step in this process. It has been shown that treatment of A549 cells with CCL19 enhanced the invasion and migratory ability of these cells, which was decreased upon exposure to siRNA-CCL19, indicating CCL19-dependent migration of A549 cells. Due to the enhanced expression of heparanase-1 during the exposure to CCL19, it was assumed that heparanase-1 is the target gene for CCL19-induced tumor cell migration. Mechanistically, it has been demonstrated that CCL19/CCR7 signaling in tumor cells upregulates the expression of a transcription factor called Sp1. This protein intrinsically binds to the GC box in gene promoters, and a ChIP assay has concluded that Sp1 is able to bind to the heparanase promoter after tumor cell incubation with CCL19 and consequently increases heparanase-1 expression. Overall, CCL19/CCR7 signaling increased heparanase-1 expression, allowing A549 tumor cells to migrate [56].
Another mechanism involved in tumor cell metastasis depends on alteration in the cytoskeletal rearrangement. Src kinase is an enzyme that is involved in several cellular activities. Phosphorylated (p)-Src interacts with various signaling proteins and contributes to diverse signal transductions. Cell migration and tumor invasion are important functions in which Src kinase plays a pivotal role. In an experiment, CCL19 administration to PCI-37B cells (an HNSCC cell line) upregulated p-Src protein levels and enhanced its activation. In addition, PCI-37B treatment with CCL19 elevated cell adhesion factors, including proline-rich tyrosine kinase 2 (Pyk2) and Paxillin in these cells [57]. Furthermore, CCL19 induced actin filaments to be woven into a complex network to form an F-actin ring or F-actin body and consequently created large flak pseudopods and invasive pseudopodia connections in these cells as CCL19-treated cells tended to be fusiform and round with reduced polar connections. Through these observations, it is believed that Src performs a crucial role in migratory changes in PCI-37B cells, CCL19-dependently [57]. Another study by Xu et al. confirmed that CCL19 triggered the RhoA/ROCK pathway in PCI-37B cells to induce Pyk2 and cofilin and initiate invasion and migration in these cells [58]. In addition to the formation of pseudopodia and morphologic changes in PCI-37B, CCL19 also induced reorganization of the actin cytoskeleton through stimulation of integrin αvβ3 phosphorylation. Activation of αvβ3 regulates cell adhesion and migration in metastatic HNSCC cells [59].
Acquisition of mesenchymal characteristics during the epithelial–mesenchymal transition (EMT) process is another proposed mechanism that contributes to tumor metastasis. In this process, epithelial cells lose adhesion, reduce cell polarity, and conversely acquire migratory and invasive abilities. Furthermore, epithelial cells decrease E-cadherin and increase N-cadherin, vimentin, fibronectin, and MMPs, which are triggered by Snail and Twist transcription factors [60]. Treatment of breast cancer cells (MCF-7 cells) with CCL19-induced AKT signaling and subsequently upregulated vimentin, N-cadherin, MMP2/9, and downregulated E-cadherin, implying that CCL19 administration confers the migratory ability to these cells, which is conversely inhibited by CCR7-siRNA or suppression of the AKT pathway [61]. In addition to breast cancer, CCL19 expression in cervical cancer cells (ME-180 and HeLa cells) was correlated to cell migration through induing vimentin, E-cadherin, and MMP2/9 which was markedly decreased after CCL19 knockdown [62]. CCL19-induced EMT in pancreatic ductal carcinoma (PDAC) was attributed to up-regulation of the Twist transcription factor, which was stimulated through the ERK/PI3K/AKT signaling pathway [63]. In epithelial ovarian cancer cells (SKOV-3 cells), it has been shown that CrkL, a member of the Crk family of adaptor proteins, contributed to CCL19-induced EMT. Upon CCL19 administration, levels of CrkL, AKT, ERK, N-cadherin, Snail, and MMP9 were increased, and the EMT process was activated in SKOV-3 cells, which was conversely diminished by CrkL knockdown, indicating that CrkL exerts a pivotal role in the CCL19-stimulated EMT process [64].
In addition to the above-mentioned mechanisms in metastasis, CCL19 also contributed to the inhibition of anoikis to promote cancer metastasis. Anoikis is a form of programmed cell death that takes place when cells lose attachment to the surrounding ECM. Therefore, inhibition of anoikis through upregulation of pro-survival factors, including Bcl-2 and Bcl-xL, is a crucial step in tumor cell detachment from originating tissue to spread in the body. Treatment of breast cancer cells (MDA-MB231 cells) significantly upregulated Bcl-xL and Bcl-2 in these cells, indicating an anti-anoikis effect of CCL19 [65].
Role of CCL19 in triggering inflammatory milieu in the tumor microenvironment
As mentioned before, many studies have shown that inflammation is intimately associated with cancer development and progression, as well as the effectiveness of anti-cancer therapy [39]. Chronic inflammation contributes to immunosuppression, resulting in a favorable microenvironment for tumorigenesis, progression, and metastasis [40]. In contrast, acute inflammation triggers an anti-tumor immune response that assists in cancer cell killing, whereas cancer-induced chronic inflammation promotes tumor cell growth and resistance to therapeutic agents [38]. Malignancy and its development have both been linked to chronic inflammation in most cancers. It has been observed that acute inflammation caused by exogenous stimulators can increase anti-tumor immunity by enhancing the maturation and activity of DCs and the induction of effector T-cells [38].
Recently, it has been confirmed that inflammation pre-disposes to cancer development and prompts tumorigenesis. Due to the plasticity of tumor cells, the formation of an inflammatory niche continuously changes the function and phenotype of these cells. It was assumed that secretion of soluble factors from triple-negative breast cancer (TNBC) in the tumor microenvironment activated the JNK/c-Jun signaling pathway in DCs to migrate toward the CCL19 gradient in this microenvironment. Tumor-infiltrated DCs upregulated proinflammatory cytokines such as IL-1β and IL-6 to induce more potent, highly proliferative, and inflammatory IFN-γ-producing T cells. Therefore, tumor-secreted factors accompanied by CCL19 manipulate DCs to build a chronic inflammatory milieu which consequently promotes tumor growth [66]. According to previous research, increased levels of IL-6 and IL-1β cytokines have been linked to tumor invasiveness and a worse prognosis in women with metastatic breast cancer [67, 68]. IL-6 can increase the proliferation of TNBCs as well as their resistance to chemotherapy and apoptosis [69]. Furthermore, tumor cells can adopt evolutionary pathways triggered by IFN-γ, which can result in the edition of tumors and selection of resistant clones, hence increasing the growth or development of the tumor [70]. Although it was claimed that CD1a+/Langerin+ DCs are the possible DC subset in this condition, more research is needed to determine the tumor-promoting DC subset in this process. These investigations suggested an innovative cancer-evading method by explaining how the soluble components of TNBCs regulate DCs in order to create dysfunctional T-cells that may contribute to tumor growth and progression [71].
CCL19 in cancer prognosis
The prognosis of cancer is the estimation of the expected course and outcome of the disease and commonly refers to the rate of successful treatment and chances of recovery. The examination or evaluation of dysregulated proteins, including growth factors, hormones, cytokines, and chemokines, can be used as a prognostic factor to elucidate the treatment process [72]. Immunohistochemistry analysis of CCL19 expression in cervical cancer cell lines (CaSki, SiHa, C33A, HeLa, and ME-180 cells) compared with its normal counterpart (H8 cells) has indicated that CCL19 expression was higher in cancerous cells. Moreover, this observation was also confirmed in 62 cervical cancer tissue samples compared with adjacent non-cancerous tissues in which CCL19 expression was positively associated with TNM stage grouping and tumor diameter, indicating the value of this chemokine as a potential target for cervical cancer therapies and survival in the future [62]. Analysis of CCL19 secretion from HNSCC tumor cells showed similar results to cervical cancer, in which CCL19 production in HNSCC tumors was higher than benign samples. Similarly, metastatic HNSCC-derived tumor cells have been shown to express a higher level of CCL19 compared with those cells from primary tumors, suggesting that CCL19 production might be a prognostic factor for predicting metastasis of HNSCC cells to lymph nodes [50].
The evaluation of CCL19 mRNA expression and its protein level in CRC tissue and cell lines compared with normal tissue and cell lines showed contradictory results to cervical cancer, as CCL19 expression negatively correlated with tumor size and invasiveness, and patients with CCL19-positive also had longer lifespans [73]. Evaluation of CCL19 mRNA expression in lung adenocarcinoma showed similar results to CRC. In this study, higher CCL19 levels in tumor samples correlated with better prognosis and a higher overall survival rate. Also, higher CCL19 expression was associated with higher recurrence-free in patients with lung adenocarcinoma, indicating a good prognostic biomarker for postoperative follow-up [74].
Besides, using a statistical regression model, also called a risk prediction model, can correlate disease outcome with various factors, especially gene expression patterns. Therefore, constructing a risk model using multiple genes might be promising in disease prediction. Concerning CCL19 and cancer, a risk model based on four immune-related genes, including IL13RA2, BIRC5, INHBE, and CCL19, has been proposed for prognostic prediction of papillary renal cell carcinoma (pRCC). This prognostic model served as a prediction for enhanced immune cell infiltration (T and B cells) with the stratification capability of patients with the pRCC in terms of different mutation burdens. In this risk prediction model, patients with pRCC with high-risk scores have upregulated the mentioned genes, whereas these genes exhibited a negative correlation with lower risk in patients with pRCC [75]. In another risk prediction model, CCL19 was accompanied by PRAME, ABCA8, APOD, and FN1 genes to discriminate breast fibroepithelial lesions, including fibroadenomas (a benign tumor) and phyllodes (a recurrent type) tumors. Discrimination of these two tumors before treatment is critical to clinical management. This predictive model showed an accuracy of 92.6%, specificity of 94.7%, and sensitivity of 82.9% for classifying breast fibroepithelial lesions into phyllodes and fibroadenomas tumors, indicating the advent of a possible valuable tool for helping pathologists in this condition [76].
Therapeutic approaches
As discussed earlier, the administration of CCL19 to the tumor microenvironment has induced strong anti-tumor responses. As a result, in tumors where CCL19 has a tumor-suppressive function, using CCL19 as an adjuvant to induce an immune response may be a promising approach in cancer immunotherapy [41]. For instance, intranodal injection of CCL19 led to a significant reduction in tumor volume and growth accompanied by marked CD4+ and CD8+ T-cell infiltration into tumor and increased secretion of anti-tumor cytokines but decreased level of TGF-β in a murine model of spontaneous bronchoalveolar cell carcinoma [77]. In addition to using recombinant CCL19 without any further formulation, it has been shown that using novel CCL19-based modalities may be beneficial in enhancing immunotherapy [78–80]. Therefore, using vehicles or an efficient delivery system would be helpful in this regard.
Recently, targeted therapy has been proposed as a high potential method in the treatment of diseases, especially cancers. These therapies are yielded by small molecular structures including proteins (e.g. cytokines and chemokines), nucleic acids (miRNAs, siRNA, etc.) incorporated into nanocarriers such as nanoliposomes. Regarding nano-liposomal CCL19-based immunotherapy, He et al. have designed a targeted gene delivery system called FDMCA-pMIP-3β comprised of 1,2-Dioleoyl-3-trimethylammonium-propane (DOTAP), folic acid-polyethylene glycol-poly lactide (FA-PEG-PCL) with a plasmid of CCL19 (MIP-3β) to treat 4T1 breast cancer-bearing mice with established lung metastasis. In vitro administration of this system to 4T1 cells increased IFN-γ and TNFα secretion from macrophages and lymphocytes while inhibiting tumor growth by up to 41%. In vivo results demonstrated that using FDMCA-pMIP-3β significantly increased IFN-γ-producing T helper cells and CTLs, decreased population of M2 macrophages, enhanced MHCII expression in DCs, and reduced tumor burden as well as metastatic nodes in mice, indicating that FDMCA-pMIP-3β efficiently enhanced immune responses to fight against tumor progression [81].
Among diverse delivery systems, cellular vehicles are preferably used to deliver genes over viral vectors due to their simplicity, non-invasive, non-deleterious manipulation, low risk of reimplantation, and adequate cellular tropism. Recently, embryonic endothelial progenitor cells (eEPCs) have been considered efficient cells that can be modified to deliver genes to the target cells [82, 83]. In a study by Hamanishi et al., eEPCs were used as a vehicle to retrovirally transduce the CCL19 gene to manipulate the tumor microenvironment and treat murine ovarian cancer through activation of local immunity. To examine the efficacy of eEPCs-CCL19 in recruiting immune cells, these cells were inoculated with tumor cells subcutaneously. Results showed that eEPCs-CCL19 induced local immunity and caused suppression of HM-1 cervical tumor and B16 melanoma growth. Moreover, higher infiltration of CD4+ and CD8+ T-cells and CD11c+ DCs was observed in tumor specimens inoculated with eEPC-CCL19. To evaluate the effects of eEPC-CCL19 on remote lung metastases, transduced cells were injected intravenously. Results demonstrated a significant reduction in the number of metastatic foci and prolonged survival in both murine models treated with eEPCs-CCL19, as this anti-tumor effectiveness was not shown in the systemic injection of recombinant CCL19, indicating that this approach might be useful for diverse malignant phenotypes [84].
Tertiary lymphoid structures (TLS) are ectopic cell aggregates that are structurally similar to secondary lymphatic organs. Unlike secondary lymphatic organs, TLS are generated in non-lymphoid tissues in response to local inflammation [85]. In some tumors, the adaptive immune response triggers TLS in situ, which requires the optimal concentration of cytokines and chemokines as well as the presence of particular immune cell types [86]. TLS development can occur both in the marginal and central regions of solid tumors, which show variable degrees of maturity. In contrast, mature TLS are composed of germinal centers and T-cell and B-cell aggregates which are similar to secondary lymphatic organs. These structures contain immune cells, including DC, macrophages, neutrophils, B cells, TCD4+ and TCD8+ lymphocytes, and plasma cells [87, 88]. Furthermore, high endothelial venules (HEVs) are frequently seen in tumors’ TLS, allowing early recruitment of immune cells as well as the release of activated immune cells from TLS into the circulation [89]. TLS have been detected in numerous tumor types, such as lung, breast, oral squamous cell carcinoma, stomach, colon, liver, bladder, sarcoma, ovarian cancer, clear cell renal cell carcinoma, and melanoma [85, 90, 91]. The formation of TLS and the cell density of TLS vary depending on the kind of tumor and the individual patient. Although the presence of TLS is strongly related to beneficial outcomes in most research, some investigations have found no such significant relationship between TLS formation and prognosis for the patient [24, 92]. These discrepancies in the researchers’ findings may be related to a number of factors, including the location of TLS formation in the tumor nucleus versus the invasive margin. It can also be related to the tumor stage, which includes primary lesions versus the metastatic stage of the tumor or the amount of mutation in the tumor. The treatment history of the patient and the diversity of cellular immune composition in TLS, especially in T- and B-cell subsets, may be a justification for these conflicting findings [92].
Approaches that boost the development of de novo TLS in patient tumors have the potential to improve the efficacy of anti-cancer therapies as well as the progression and survival rates of patients. Some pre-clinical studies have shown the potential efficacy of such therapies, but additional clinical trials may be useful in determining the therapeutic value of combining targeted TLS cellular composition with immunotherapy. Given that CCL19 plays a critical role in both the recruitment of immune cells to TLS and the interaction of these cells with each other, the researchers chose to take advantage of this to recruit immune cells to the tumor location. By increasing the possibility of interaction between DC, T cells, and B cells, CCL19 has demonstrated great promise as a candidate for cancer therapy. Tumor-infiltrating immune cells are essential for the efficacy of immunotherapy in cancer, so the local synthesis of CCL19 by MSCs may facilitate the infiltration of these cells and the exertion of an antitumor impact in patients with cancer [93]. To summarize, findings imply that using iMSC/CCL19 in conjunction with anti-PD-L1 antibody treatment can reduce tumor growth by increasing the number of IFN-γ+/CD8+ T cells while simultaneously increasing the number of recruited CCR7+ DC into tumor locations [93]. A folic acid-modified targeted gene-delivery system consisting of the F-DMA (FA-PEG-PLA)/CCL19 complex administered to tumor-bearing mice showed significant cancer growth repression, suggesting that this approach could be useful in the treatment of cancer. The anti-cancer mechanism is formed by decreasing tumor cell proliferation through stimulating the immune system, suppressing neovascularization, and increasing apoptosis [94].
As a potential anti-cancer treatment, immune checkpoint blockade (ICB) antibody therapy has recently gained interest [95, 96]. Patients with specific types of cancer may benefit from anti-cancer effects from ICB antibodies targeting programmed death-1 (PD-1), PD-1 ligand (PD-L1), and cytotoxic T-lymphocyte associated protein 4 (CTLA4) [97]. The existence of antitumor T-cells in tumor tissues is critical for ICB treatment because it restores exhausted antitumor T-cells in tumor locations when targeting PD-1 and PD-L1. According to the studies, T-cell infiltration in tumor locations is associated with the response of anti-cancer immunotherapy [93]. Recently, a study was conducted on patients with advanced urothelial cancer. These patients received a combination of anti-PD-1 and anti-CTLA-4 antibodies prior to tumor removal. In this investigation, there was no correlation between the existence of TLS at baseline and the response to therapy. However, all patients who exhibited full pathological responses had higher TLS levels after the therapy. This means that TLS can be produced during immune checkpoint treatment, which will aid in creating anti-tumor immune responses at the site of the tumor [98]. In another study, an RGD-DMA/pCCL19-BMS-1 system was utilized to co-deliver the TLS-stimulating chemokine CCL19-encoding plasmid DNA (CCL19 pDNA) with the PD-L1 inhibitor (BMS-1). Not only did the RGD-DMA/pCCL19-BMS-1 combination significantly suppress tumor development but it also elicited locally elevated levels of immunostimulatory cytokines at tumor sites without eliciting a systemic inflammatory response. The immunosuppressive tumor microenvironment was efficaciously altered by coadministration of RGD-DMA/pCCL19 and BMS-1, as shown by activated T lymphocytes, enhanced intratumoral infiltration of mature DCs, and macrophage repolarization from protumoral M2-phenotype to tumoricidal M1-phenotype. Higher PD-L1 expression at tumor sites as a result of increased IFN-γ levels after immunostimulatory gene therapy further indicated BMS-1’s synergistic benefits in counteracting PD-L1’s suppressive function in antitumor immunity. Thus, the combination of immunostimulatory treatment with immune checkpoint inhibitors that target several immune regulatory pathways synergistically has tremendous promise as a new immunotherapy strategy [99].
In contrast to the above-mentioned approaches in which CCL19 administration reduces tumor burden, in some cancers, inhibition of CCL19 is the aim of the therapy to suppress tumor progression. As discussed earlier, in B-cell lymphoblastic leukemia, CCL19 overexpression was positively correlated to disease development. Therefore, using the CCL19 antagonist might provide a promising result against cancer. Lurbinectedin is a synthetic alkaloid analog that exerts antineoplastic activity through binding to the minor groove of DNA and inhibition of RNA polymerase II activity, possibly resulting in cell cycle arrest. Administration of lurbinectedin to B-CLL cells decreased CCR7 expression in these cells and decreased their migration toward CCL19 and CCL21. This inhibition of migration might be the reason for the suppression of cancer cell growth [100]. β-caryophyllene (BCP) is a natural anti-inflammatory bicyclic sesquiterpene found in essential oils, which is proposed as an anti-melanoma agent. BCP feeding of B16F10 melanoma-bearing mice inhibited the CCL19/CCR7 axis, downregulated CCL19 expression, and suppressed inflammation-induced tumor growth [101].
Because CCR7 is the cognate receptor for CCL19 and CCL19 function depends on CCR7 expression on tumor cells, inhibiting CCR7 signaling or blocking its function may be identified as a novel approach in cancers where CCL19 interaction with CCR7 exerts tumor-promoting function. In this term, siRNA-dependent silencing of CCR7 in cancer cells might be promising in suppressing the growth, migration, and invasion of tumor cells [102]. Another proposed mechanism for this approach is blocking the interaction of CCR7 and CCL19. It has been found that CCR7 is a sialylated receptor protein. Overexpression of a sialylation enzyme (i.e. α-2,3-sialyltransferase) and subsequent aberrant sialylation of CCR7 in breast tumor cell lines and tissues has been shown to encourage tumor growth. Therefore, using sialidase agents or sialyltransferase inhibitors, such as AL10, inhibited CCL19-induced tumor growth [65]. Neutralizing or blocking antibodies have also been proposed to inhibit the CCR7/CCL19 interaction. It has been shown that anti-CCR7 antibodies block CCR7/CCL19 interaction and limit lymphoma cell migration, suggesting this approach as a promising method to inhibit CLL invasion into the CNS [103]. Blocking CCR7 signaling is also suggested for inhibition of CCL19-promoted resistance of tumor cells. In this regard, it has been demonstrated that inhibiting Akt-dependent signaling may improve the efficacy of EGFR- and platinum-based therapies to overcome cisplatin resistance in HNSCC [50].
Conclusion and future perspectives
CCL19 is classified into CC chemokine subtypes, whose primary function is mediating leukocyte trafficking between lymph nodes. In addition to hemostatic and physiologic functions, it has been shown that this chemokine plays a pivotal role in cancer development. Since CCL19 contributes to the trafficking of immune cells such as DCs, T cells, and NK cells, it has been confirmed that this chemokine promotes anti-tumor immune functions, hence suppressing tumor development. On the other hand, CCL19 interaction with its receptor on tumor cells induced tumor heterogeneity, inhibited cell apoptosis, and promoted tumor cell migration and metastasis (i.e. EMT), resulting in tumor development. Therefore, CCL19 shows deceptive behavior in cancer, either tumor-suppressive or tumor-supportive roles. Since CCL19 enhances immune responses in several cancers where CCL19 acts as a tumor-suppressive factor, it is proposed that this chemokine can be used as an adjuvant for immunotherapies. In CCL19-induced tumor development, downregulation of its expression or blockade of CCL19/CCR7 interaction might be helpful to treat cancer. Moreover, CCL19 dysregulation might be a prognostic biomarker to predict cancer progression and recurrence.
Despite tumor-suppressive roles, many studies regarding the tumor-supportive role of CCL19 have been implemented in vitro. Therefore, it is proposed to expand the examination of CCL19-induced tumor promotion to in vivo studies. In addition, underlying mechanisms and detailed signaling pathways should be elucidated in the tumor-suppressive role of CCL19. CCL19-induced and CCL21-induced tumor development also should be distinguished to clarify which chemokine is involved in this process. As discussed earlier, CCL19 has been proposed as a potent adjuvant in immunotherapies for animal models. Therefore, it is suggested that using CCL19 as an adjuvant with or without formulations and modalities in the clinic might be promising. Moreover, CCL19 can be formulated with other carriers or nanomaterials to enhance its efficacy for immunotherapies. Furthermore, CCL19 can be accompanied by various immunotherapeutic approaches such as chimeric antigen receptor T cell (CAR-T), ICB therapies, or other cancer vaccines. Also, CCL19 has been shown to be dysregulated in various cancers. A few cancers have been monitored for dysregulation of this chemokine to be considered a biomarker. However, it is proposed that CCL19 should be evaluated in the tumor microenvironment, lymph nodes, mononuclear cells to determine its application for being a biomarker for predicting tumor stage, metastasis, and survival. A combination of CCL19 with other specific previously determined biomarkers might help to increase their specificity and accuracy. Collectively, CCL19 is a hemostatic chemokine that should be further investigated for its contribution to cancer development. Future studies might provide new horizons and broaden our knowledge about cancer biology.
Acknowledgments
None.
Glossary
Abbreviations
- ABCA8
ATP binding cassette subfamily A member 8
- ACKR
atypical chemokine receptor
- AIM2
absent in melanoma 2
- Akt
protein kinase B
- ALL
acute leukemia lymphoma
- APCs
antigen-presenting cells
- APOD
apolipoprotein D
- Bcl-2
B cell lymphoma 2
- BIRC5
baculoviral IAP repeat containing 5
- CAR-T
chimeric antigen receptor T cell
- CCL
CC motif chemokine ligand
- CCR
CC motif chemokine receptor
- Cdc42
cell division cycle 42
- ChIP
Chromatin immunoprecipitation
- CLL
chronic leukemia lymphoma
- CNS
central nervous system
- CRAM
chemokine receptor on activated macrophages
- CRC
colorectal cancer
- CrkL
CRK like proto-oncogene
- CSF
cerebrospinal fluid
- CTLA4
cytotoxic T-lymphocyte associated protein 4
- CTLs
cytotoxic T cells
- CXCL
CXC motif chemokine ligand
- DCs
dendritic cells
- ECM
extracellular matrix
- eEPCs
embryonic endothelial progenitor cells
- EGFR
epidermal growth factor receptor
- ELC
EBI1-ligand chemokine
- Elk-1
ETS transcription factor
- EMT
epithelial-mesenchymal transition
- ERK
extracellular signal regulated kinase
- FN1
fibronectin 1
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GPCR
G protein-coupled receptor
- GSK3
glycogen synthase kinase-3
- HERVs
human endogenous retroviruses
- HEV
high endothelial venules
- HIF-1α
hypoxia-inducible factor-1α
- HNSCC
head and neck squamous cell carcinomas
- ICB
immune checkpoint blockade
- IFN-γ
interferon-γ
- iMSC
induced mesenchymal stromal cells
- INHBE
inhibin subunit beta E
- MIP3-β
macrophage proinflammatory human 3-β
- MLC
myosin light chain
- MMPs
matrix metalloproteinases
- mTOR
mammalian target of rapamycin
- NF-κB
nuclear factor-κB
- NK
natural killer
- NSCLC
non-small-cell lung carcinoma
- p70S6K
ribosomal protein S6 kinase beta-1
- PD-L1
programmed death-ligand 1
- PEG10
paternally expressed gene 10
- PGE2
prostaglandin E2
- PI3K
phosphoinositide 3-kinases
- PKC
protein kinase C
- PRAME
preferentially expressed antigen in melanoma
- Pyk2
proline-rich tyrosine kinase 2
- ROCK
Rho-associated protein kinase
- RUNX3
runt-related transcription factor 3
- S1P1
sphingosine-1-phosphate receptor 1
- SAPK
JNK1 or Janus kinase 1
- STAT3
signal transducer and activator of transcription 3
- TGFβ
transforming growth factor-β
- TLS
tertiary lymphoid structures
- TNBC
triple-negative breast cancer
- TNFα
tumor necrosis factor α
- VEGFA
vascular endothelial growth factor A
- ZAP-70
zeta-chain-associated protein kinase 70
Funding
None.
Disclosures
The authors declare no conflicts of interest.
Author contributions
Conceptualization, investigation, writing – original draft, methodology: AGS; Investigation, writing – review and editing, methodology: ZMJA-O; Investigation, writing – review and editing: Heshu Sulaiman Rahman. Investigation, writing – review and editing, methodology: Walid Kamal Abdelbasset; Investigation, writing – review and editing: Wanich Suksatan; Investigation, writing – review and editing: Dmitry O. Bokov; Investigation, methodology, writing – review and editing: Lakshmi Thangavelu; Investigation, writing – original draft, writing – review and editing: Abduladheem Turki Jalil; Investigation, writing – original draft, writing – review and editing: Farhad Jadidi-Niaragh; Investigation, writing – original draft, writing – review and editing: Hamed Mohammadi; Investigation, writing – original draft, writing - review and editing: Kazem Mashayekhi; Conceptualization, writing – original draft, writing - review and editing, methodology, supervision: Jamshid Gholizadeh Navashenaq.
Data Availability
Not Applicable.
References
- 1. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A.. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009, 30, 1073–81. [DOI] [PubMed] [Google Scholar]
- 2. Bhat AA, Nisar S, Maacha S, Carneiro-Lobo TC, Akhtar S, Siveen KS, et al. . Cytokine-chemokine network driven metastasis in esophageal cancer; promising avenue for targeted therapy. Mol Cancer 2021, 20, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shabgah AG, Al-Qaim ZH, Markov A, Yumashev AV, Ezzatifar F, Ahmadi M, et al. Chemokine CXCL14; a double-edged sword in cancer development. Int Immunopharmacol 2021, 97, 107681. [DOI] [PubMed] [Google Scholar]
- 4. Shabgah AG, Qasim MT, Mostafavi SM, Zekiy AO, Ezzatifar F, Ahmadi M, et al. CXC chemokine ligand 16: a Swiss army knife chemokine in cancer. Exp Rev Mol Med 2021, 23, e4. [DOI] [PubMed] [Google Scholar]
- 5. Link A, Vogt TK, Favre S, Britschgi MR, Acha-Orbea H, Hinz B, et al. . Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat Immunol 2007, 8, 1255–65. [DOI] [PubMed] [Google Scholar]
- 6. Baggiolini M. Chemokines in pathology and medicine. J Intern Med 2001, 250, 91–104. [DOI] [PubMed] [Google Scholar]
- 7. Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A.. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3alpha and MIP-3beta. J Immunol 1997, 158, 1033–6. [PubMed] [Google Scholar]
- 8. Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, et al. . Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem 1997, 272, 13803–9. [DOI] [PubMed] [Google Scholar]
- 9. Kawashima D, Oshitani N, Jinno Y, Watanabe K, Nakamura S, Higuchi K, et al. . Augmented expression of secondary lymphoid tissue chemokine and EBI1 ligand chemokine in Crohn’s disease. J Clin Pathol 2005, 58, 1057–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma N, Benechet AP, Lefrançois L, Khanna KM.. CD8 T cells enter the splenic T cell zones independently of CCR7, but the subsequent expansion and trafficking patterns of effector T cells after infection are dysregulated in the absence of CCR7 Migratory cues. J Immunol 2015, 195, 5227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Comerford I, Milasta S, Morrow V, Milligan G, Nibbs R.. The chemokine receptor CCX-CKR mediates effective scavenging of CCL19 in vitro. Eur J Immunol 2006, 36, 1904–16. [DOI] [PubMed] [Google Scholar]
- 12. Leick M, Catusse J, Follo M, Nibbs RJ, Hartmann TN, Veelken H, et al. . CCL19 is a specific ligand of the constitutively recycling atypical human chemokine receptor CRAM-B. Immunology 2010, 129, 536–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Purvanov V, Matti C, Samson GP, Kindinger I, Legler DF.. Fluorescently tagged CCL19 and CCL21 to monitor CCR7 and ACKR4 functions. Int J Mol Sci 2018, 19, 3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffith JW, Sokol CL, Luster AD.. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014, 32, 659–702. [DOI] [PubMed] [Google Scholar]
- 15. Nandagopal S, Wu D, Lin F.. Combinatorial guidance by CCR7 ligands for T lymphocytes migration in co-existing chemokine fields. PLoS One 2011, 6, e18183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, et al. . Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol 2002, 169, 424–33. [DOI] [PubMed] [Google Scholar]
- 17. Artinger M, Matti C, Gerken OJ, Veldkamp CT, Legler DF.. A versatile toolkit for semi-automated production of fluorescent chemokines to study CCR7 expression and functions. Int J Mol Sci 2021, 22, 4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moschovakis GL, Bubke A, Friedrichsen M, Ristenpart J, Back JW, Falk CS, et al. . The chemokine receptor CCR7 is a promising target for rheumatoid arthritis therapy. Cell Mol Immunol 2019, 16, 791–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alon R, Feigelson SW.. Chemokine signaling to lymphocyte integrins under shear flow. Microcirculation 2009, 16, 3–16. [DOI] [PubMed] [Google Scholar]
- 20. Laufer JM, Legler DF.. Beyond migration-chemokines in lymphocyte priming, differentiation, and modulating effector functions. J Leukoc Biol 2018, 104, 301–12. [DOI] [PubMed] [Google Scholar]
- 21. Robertson MJ, Williams BT, Christopherson K 2nd, Brahmi Z, Hromas R.. Regulation of human natural killer cell migration and proliferation by the exodus subfamily of CC chemokines. Cell Immunol 2000, 199, 8–14. [DOI] [PubMed] [Google Scholar]
- 22. Yan Y, Zhao W, Liu W, Li Y, Wang X, Xun J, et al. . CCL19 enhances CD8+ T-cell responses and accelerates HBV clearance. J Gastroenterol 2021, 56, 769–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anderson CA, Solari R, Pease JE.. Biased agonism at chemokine receptors: obstacles or opportunities for drug discovery? J Leukoc Biol 2016, 99, 901–9. [DOI] [PubMed] [Google Scholar]
- 24. Hjortø GM, Larsen O, Steen A, Daugvilaite V, Berg C, Fares S, et al. . Differential CCR7 targeting in dendritic cells by three naturally occurring CC-chemokines. Front Immunol 2016, 7, 568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan Y, Chen R, Wang X, Hu K, Huang L, Lu M, et al. . CCL19 and CCR7 expression, signaling pathways, and adjuvant functions in viral infection and prevention. Front Cell Dev Biol 2019, 7, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brandum EP, Jørgensen AS, Rosenkilde MM, Hjortø GM.. Dendritic cells and CCR7 expression: an important factor for autoimmune diseases, chronic inflammation, and cancer. Int J Mol Sci. 2021, 22, 8340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shannon LA, McBurney TM, Wells MA, Roth ME, Calloway PA, Bill CA, et al. . CCR7/CCL19 controls expression of EDG-1 in T cells. J Biol Chem 2012, 287, 11656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Raju R, Gadakh S, Gopal P, George B, Advani J, Soman S, et al. . Differential ligand-signaling network of CCL19/CCL21-CCR7 system. Database 2015, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kohout TA, Nicholas SL, Perry SJ, Reinhart G, Junger S, Struthers RS.. Differential desensitization, receptor phosphorylation, β-arrestin recruitment, and ERK1/2 activation by the two endogenous ligands for the CC chemokine receptor 7∗. J Biol Chem 2004, 279, 23214–22. [DOI] [PubMed] [Google Scholar]
- 30. Zhen-jin Z, Peng L, Fa-yu L, Liyan S, Chang-fu S.. PKCα take part in CCR7/NF-κB autocrine signaling loop in CCR7-positive squamous cell carcinoma of head and neck. Mol Cell Biochem 2011, 357, 181–7. [DOI] [PubMed] [Google Scholar]
- 31. Zhao ZJ, Liu FY, Li P, Ding X, Zong ZH, Sun CF.. CCL19-induced chemokine receptor 7 activates the phosphoinositide-3 kinase-mediated invasive pathway through Cdc42 in metastatic squamous cell carcinoma of the head and neck. Oncol Rep 2011, 25, 729–37. [DOI] [PubMed] [Google Scholar]
- 32. Liu FY, Zhao ZJ, Li P, Ding X, Zong ZH, Sun CF.. Mammalian target of rapamycin (mTOR) is involved in the survival of cells mediated by chemokine receptor 7 through PI3K/Akt in metastatic squamous cell carcinoma of the head and neck. Br J Oral Maxillofac Surg 2010, 48, 291–6. [DOI] [PubMed] [Google Scholar]
- 33. Cuesta-Mateos C, López-Giral S, Alfonso-Pérez M, de Soria VG, Loscertales J, Guasch-Vidal S, et al. . Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp Hematol 2010, 38, 756–64, 764.e1–4. [DOI] [PubMed] [Google Scholar]
- 34. Richardson SJ, Matthews C, Catherwood MA, Alexander HD, Carey BS, Farrugia J, et al. . ZAP-70 expression is associated with enhanced ability to respond to migratory and survival signals in B-cell chronic lymphocytic leukemia (B-CLL). Blood 2006, 107, 3584–92. [DOI] [PubMed] [Google Scholar]
- 35. Nguyen AV, Wu YY, Liu Q, Wang D, Nguyen S, Loh R, et al. . STAT3 in epithelial cells regulates inflammation and tumor progression to malignant state in colon. Neoplasia 2013, 15, 998–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kudo-Saito C, Yura M, Yamamoto R, Kawakami Y.. Induction of immunoregulatory CD271+ cells by metastatic tumor cells that express human endogenous retrovirus H. Cancer Res 2014, 74, 1361–70. [DOI] [PubMed] [Google Scholar]
- 37. Kim HJ, Park J, Lee SK, Kim KR, Park KK, Chung WY.. Loss of RUNX3 expression promotes cancer-associated bone destruction by regulating CCL5, CCL19 and CXCL11 in non-small cell lung cancer. J Pathol 2015, 237, 520–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao H, Wang TC, Li XF, Zhang NN, Li L, Zhou C, et al. . Long-term stability and protection efficacy of the RBD-targeting COVID-19 mRNA vaccine in nonhuman primates. Signal Transduct Target Ther 2021, 6, 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crusz SM, Balkwill FR.. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015, 12, 584–96. [DOI] [PubMed] [Google Scholar]
- 40. Schaue D, Micewicz ED, Ratikan JA, Xie MW, Cheng G, McBride WH (eds). Radiation and Inflammation. Seminars in Radiation Oncology. Elsevier,2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hillinger S, Yang SC, Zhu L, Huang M, Duckett R, Atianzar K, et al. . EBV-induced molecule 1 ligand chemokine (ELC/CCL19) promotes IFN-gamma-dependent antitumor responses in a lung cancer model. J Immunol 2003, 171, 6457–65. [DOI] [PubMed] [Google Scholar]
- 42. Lu J, Ma J, Cai W, Wangpu X, Feng H, Zhao J, et al. . CC motif chemokine ligand 19 suppressed colorectal cancer in vivo accompanied by an increase in IL-12 and IFN-γ. Biomed Pharmacother 2015, 69, 374–9. [DOI] [PubMed] [Google Scholar]
- 43. Braun SE, Chen K, Foster RG, Kim CH, Hromas R, Kaplan MH, et al. The CC Chemokine CKβ-11/MIP-3β/ELC/Exodus 3 mediates tumor rejection of murine breast cancer cells through NK cells. J Immunol 2000, 164, 4025–31. [DOI] [PubMed] [Google Scholar]
- 44. Zhou R, Sun J, He C, Huang C, Yu H.. CCL19 suppresses gastric cancer cell proliferation, migration, and invasion through the CCL19/CCR7/AIM2 pathway. Hum Cell 2020, 33, 1120–32. [DOI] [PubMed] [Google Scholar]
- 45. Xu Z, Zhu C, Chen C, Zong Y, Feng H, Liu D, et al. . CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis 2018, 9, 974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dagogo-Jack I, Shaw AT.. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol 2018, 15, 81–94. [DOI] [PubMed] [Google Scholar]
- 47. Marusyk A, Almendro V, Polyak K.. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer 2012, 12, 323–34. [DOI] [PubMed] [Google Scholar]
- 48. Kim BJ, Hannanta-Anan P, Ryd A, Swartz MA, Wu M.. Lymphoidal chemokine CCL19 promoted the heterogeneity of the breast tumor cell motility within a 3D microenvironment revealed by a Lévy distribution analysis. Integr Biol (Camb) 2020, 12, 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pfeffer CM, Singh ATK.. Apoptosis: a target for anticancer therapy. Int J Mol Sci. 2018, 19, 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang J, Seethala RR, Zhang Q, Gooding W, van Waes C, Hasegawa H, et al. . Autocrine and paracrine chemokine receptor 7 activation in head and neck cancer: implications for therapy. J Natl Cancer Inst 2008, 100, 502–12. [DOI] [PubMed] [Google Scholar]
- 51. Okabe H, Satoh S, Furukawa Y, Kato T, Hasegawa S, Nakajima Y, et al. . Involvement of PEG10 in human hepatocellular carcinogenesis through interaction with SIAH1. Cancer Res 2003, 63, 3043–8. [PubMed] [Google Scholar]
- 52. Chunsong H, Yuling H, Li W, Jie X, Gang Z, Qiuping Z, et al. . CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+CD5+ B Cells. J Immunol 2006, 177, 6713. [DOI] [PubMed] [Google Scholar]
- 53. Wang X, Yuling H, Yanping J, Xinti T, Yaofang Y, Feng Y, et al. CCL19 and CXCL13 synergistically regulate interaction between B cell acute lymphocytic leukemia CD23+CD5+ B cells and CD8+ T cells. J Immunol 2007, 179, 2880. [DOI] [PubMed] [Google Scholar]
- 54. O’Connor T, Zhou X, Kosla J, Adili A, Garcia Beccaria M, Kotsiliti E, et al. . Age-Related gliosis promotes central nervous system lymphoma through ccl19-mediated tumor cell retention. Cancer Cell 2019, 36, 250–267.e9. [DOI] [PubMed] [Google Scholar]
- 55. Buonamici S, Trimarchi T, Ruocco MG, Reavie L, Cathelin S, Mar BG, et al. . CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 2009, 459, 1000–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhang Q, Sun L, Yin L, Ming J, Zhang S, Luo W, et al. . CCL19/CCR7 upregulates heparanase via specificity protein-1 (Sp1) to promote invasion of cell in lung cancer. Tumour Biol 2013, 34, 2703–8. [DOI] [PubMed] [Google Scholar]
- 57. Xu H, Yang X, Zhang Q, Chen L.. Effects of CCR7 and Src on invasion and migration of salivary gland tumor. Eur Rev Med Pharmacol Sci 2019, 23, 3813–20. [DOI] [PubMed] [Google Scholar]
- 58. Xu Z, Zheng X, Yang L, Liu F, Zhang E, Duan W, et al. . Chemokine receptor 7 promotes tumor migration and invasiveness via the RhoA/ROCK pathway in metastatic squamous cell carcinoma of the head and neck. Oncol Rep 2015, 33, 849–55. [DOI] [PubMed] [Google Scholar]
- 59. Li P, Liu F, Sun L, Zhao Z, Ding X, Shang D, et al. . Chemokine receptor 7 promotes cell migration and adhesion in metastatic squamous cell carcinoma of the head and neck by activating integrin αvβ3. Int J Mol Med 2011, 27, 679–87. [DOI] [PubMed] [Google Scholar]
- 60. Kotiyal S, Bhattacharya S.. Events of Molecular Changes in Epithelial-Mesenchymal Transition. Crit Rev Eukaryot Gene Expr 2016, 26, 163–71. [DOI] [PubMed] [Google Scholar]
- 61. Xu B, Zhou M, Qiu W, Ye J, Feng Q.. CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial-mesenchymal transition and suppressing apoptosis through AKT pathway. Cancer Med 2017, 6, 1062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang X, Wang Y, Cao Y, Zhang X, Zhao H.. Increased CCL19 expression is associated with progression in cervical cancer. Oncotarget 2017, 8, 73817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Li K, Xu B, Xu G, Liu R.. CCR7 regulates Twist to induce the epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma. Tumour Biol 2016, 37, 419–24. [DOI] [PubMed] [Google Scholar]
- 64. Cheng S, Guo J, Yang Q, Yang X.. Crk-like adapter protein regulates CCL19/CCR7-mediated epithelial-to-mesenchymal transition via ERK signaling pathway in epithelial ovarian carcinomas. Med Oncol 2015, 32, 47. [DOI] [PubMed] [Google Scholar]
- 65. Su ML, Chang TM, Chiang CH, Chang HC, Hou MF, Li WS, et al. . Inhibition of chemokine (C-C motif) receptor 7 sialylation suppresses CCL19-stimulated proliferation, invasion and anti-anoikis. PLoS One 2014, 9, e98823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hwang H, Shin C, Park J, Kang E, Choi B, Han JA, et al. . Human breast cancer-derived soluble factors facilitate CCL19-induced chemotaxis of human dendritic cells. Sci Rep 2016, 6, 30207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DeNardo DG, Coussens LM.. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res 2007, 9, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pantschenko AG, Pushkar I, Anderson KH, Wang Y, Miller LJ, Kurtzman SH, et al. . The interleukin-1 family of cytokines and receptors in human breast cancer: implications for tumor progression. Int J Oncol 2003, 23, 269–84. [PubMed] [Google Scholar]
- 69. Hartman ZC, Poage GM, den Hollander P, Tsimelzon A, Hill J, Panupinthu N, et al. . Growth of triple-negative breast cancer cells relies upon coordinate autocrine expression of the proinflammatory cytokines IL-6 and IL-8. Cancer Res 2013, 73, 3470–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Matsushita H, Vesely MD, Koboldt DC, Rickert CG, Uppaluri R, Magrini VJ, et al. . Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature 2012, 482, 400–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Hwang H, Shin C, Park J, Kang E, Choi B, Han J-A, et al. Human breast cancer-derived soluble factors facilitate CCL19-induced chemotaxis of human dendritic cells. Scientific reports. 2016, 6, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gowhari Shabgah A, Amir A, Gardanova ZR, Olegovna Zekiy A, Thangavelu L, Ebrahimi Nik M, et al. . Interleukin-25: New perspective and state-of-the-art in cancer prognosis and treatment approaches. Cancer Med 2021, 10, 5191–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lu J, Zhao J, Feng H, Wang P, Zhang Z, Zong Y, et al. . Antitumor efficacy of CC motif chemokine ligand 19 in colorectal cancer. Dig Dis Sci 2014, 59, 2153–62. [DOI] [PubMed] [Google Scholar]
- 74. Itakura M, Terashima Y, Shingyoji M, Yokoi S, Ohira M, Kageyama H, et al. . High CC chemokine receptor 7 expression improves postoperative prognosis of lung adenocarcinoma patients. Br J Cancer 2013, 109, 1100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wang L, Yang Z, Wang C, Zhu X, Shi J, Niu N.. Construction of a prognostic value model in papillary renal cell carcinoma by immune-related genes. Medicine (Baltimore) 2021, 100, e24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Tan WJ, Cima I, Choudhury Y, Wei X, Lim JC, Thike AA, et al. . A five-gene reverse transcription-PCR assay for pre-operative classification of breast fibroepithelial lesions. Breast Cancer Res 2016, 18, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hillinger S, Yang SC, Batra RK, Strieter RM, Weder W, Dubinett SM, et al. . CCL19 reduces tumour burden in a model of advanced lung cancer. Br J Cancer 2006, 94, 1029–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nguyen-Hoai T, Baldenhofer G, Ahmed MS, Pham-Duc M, Gries M, Lipp M, et al. . CCL19 (ELC) improves TH1-polarized immune responses and protective immunity in a murine Her2/neu DNA vaccination model. J Gene Med 2012, 14, 128–37. [DOI] [PubMed] [Google Scholar]
- 79. Nguyen-Hoai T, Hohn O, Vu MD, Baldenhofer G, Sayed Ahmed MS, Dörken B, et al. . CCL19 as an adjuvant for intradermal gene gun immunization in a Her2/neu mouse tumor model: improved vaccine efficacy and a role for B cells as APC. Cancer Gene Ther 2012, 19, 880–7. [DOI] [PubMed] [Google Scholar]
- 80. Hou JM, Zhao X, Tian L, Li G, Zhang R, Yao B, et al. . Immunotherapy of tumors with recombinant adenovirus encoding macrophage inflammatory protein 3β induces tumor-specific immune response in immunocompetent tumor-bearing mice. Acta Pharmacol Sin 2009, 30, 355–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. He Y, Wang M, Li X, Yu T, Gao X.. Targeted MIP-3β plasmid nanoparticles induce dendritic cell maturation and inhibit M2 macrophage polarisation to suppress cancer growth. Biomaterials 2020, 249, 120046. [DOI] [PubMed] [Google Scholar]
- 82. Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, et al. . Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell 2004, 5, 477–88. [DOI] [PubMed] [Google Scholar]
- 83. Gómez-Navarro J, Contreras JL, Arafat W, Jiang XL, Krisky D, Oligino T, et al. . Genetically modified CD34+ cells as cellular vehicles for gene delivery into areas of angiogenesis in a rhesus model. Gene Ther 2000, 7, 43–52. [DOI] [PubMed] [Google Scholar]
- 84. Hamanishi J, Mandai M, Matsumura N, Baba T, Yamaguchi K, Fujii S, et al. . Activated local immunity by CC chemokine ligand 19-transduced embryonic endothelial progenitor cells suppresses metastasis of murine ovarian cancer. Stem Cells 2010, 28, 164–73. [DOI] [PubMed] [Google Scholar]
- 85. Jacquelot N, Tellier J, Nutt Sl BG.. Tertiary lymphoid structures and B lymphocytes in cancer prognosis and response to immunotherapies. Oncoimmunology 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pitzalis C, Jones GW, Bombardieri M, Jones SA.. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol 2014, 14, 447–62. [DOI] [PubMed] [Google Scholar]
- 87. Sharonov GV, Serebrovskaya EO, Yuzhakova DV, Britanova OV, Chudakov DM.. B cells, plasma cells and antibody repertoires in the tumour microenvironment. Nat Rev Immunol 2020, 20, 294–307. [DOI] [PubMed] [Google Scholar]
- 88. Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH.. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer 2019, 19, 307–25. [DOI] [PubMed] [Google Scholar]
- 89. Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, et al. . High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012, 1, 829–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Tokunaga R, Nakagawa S, Sakamoto Y, Nakamura K, Naseem M, Izumi D, et al. . 12-Chemokine signature, a predictor of tumor recurrence in colorectal cancer. Int J Cancer 2020, 147, 532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, et al. . Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011, 71, 6391–9. [DOI] [PubMed] [Google Scholar]
- 92. Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, et al. . Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014, 74, 705–15. [DOI] [PubMed] [Google Scholar]
- 93. Nishino M, Ramaiya NH, Hatabu H, Hodi FS.. Monitoring immune-checkpoint blockade: response evaluation and biomarker development. Nat Rev Clin Oncol 2017, 14, 655–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Liu X, Wang B, Li Y, Hu Y, Li X, Yu T, et al. . Powerful anticolon tumor effect of targeted gene immunotherapy using folate-modified nanoparticle delivery of CCL19 to activate the immune system. ACS Cent Sci 2019, 5, 277–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Chiou VL, Burotto M.. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015, 33, 3541–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Nishino M, Hatabu H, Johnson BE, McLoud TC.. State of the art: response assessment in lung cancer in the era of genomic medicine. Radiology 2014, 271, 6–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nishino M. Immune-related response evaluations during immune-checkpoint inhibitor therapy: establishing a “common language” for the new arena of cancer treatment. J Immunother Cancer 2016, 4, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. van Dijk N, Gil-Jimenez A, Silina K, Hendricksen K, Smit LA, de Feijter JM, et al. . Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med 2020, 26, 1839–44. [DOI] [PubMed] [Google Scholar]
- 99. Yu T, Nie W, Hong Z, He Y, Chen J, Mi X, et al. . Synergy of immunostimulatory genetherapy with immune checkpoint blockade motivates immune response to eliminate cancer. Adv Funct Mater 2021;31, 2100715. [Google Scholar]
- 100. Risnik D, Colado A, Podaza E, Almejún MB, Elías EE, Bezares RF, et al. . Immunoregulatory effects of lurbinectedin in chronic lymphocytic leukemia. Cancer Immunol Immunother 2020, 69, 813–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Jung JI, Kim EJ, Kwon GT, Jung YJ, Park T, Kim Y, et al. . β-Caryophyllene potently inhibits solid tumor growth and lymph node metastasis of B16F10 melanoma cells in high-fat diet-induced obese C57BL/6N mice. Carcinogenesis 2015, 36, 1028–39. [DOI] [PubMed] [Google Scholar]
- 102. Chi BJ, Du CL, Fu YF, Zhang YN, Wang RW.. Silencing of CCR7 inhibits the growth, invasion and migration of prostate cancer cells induced by VEGFC. Int J Clin Exp Pathol 2015, 8, 12533–40. [PMC free article] [PubMed] [Google Scholar]
- 103. Alfonso-Pérez M, López-Giral S, Quintana NE, Loscertales J, Martín-Jiménez P, Muñoz C.. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J Leukoc Biol 2006, 79, 1157–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.