Figure 2.
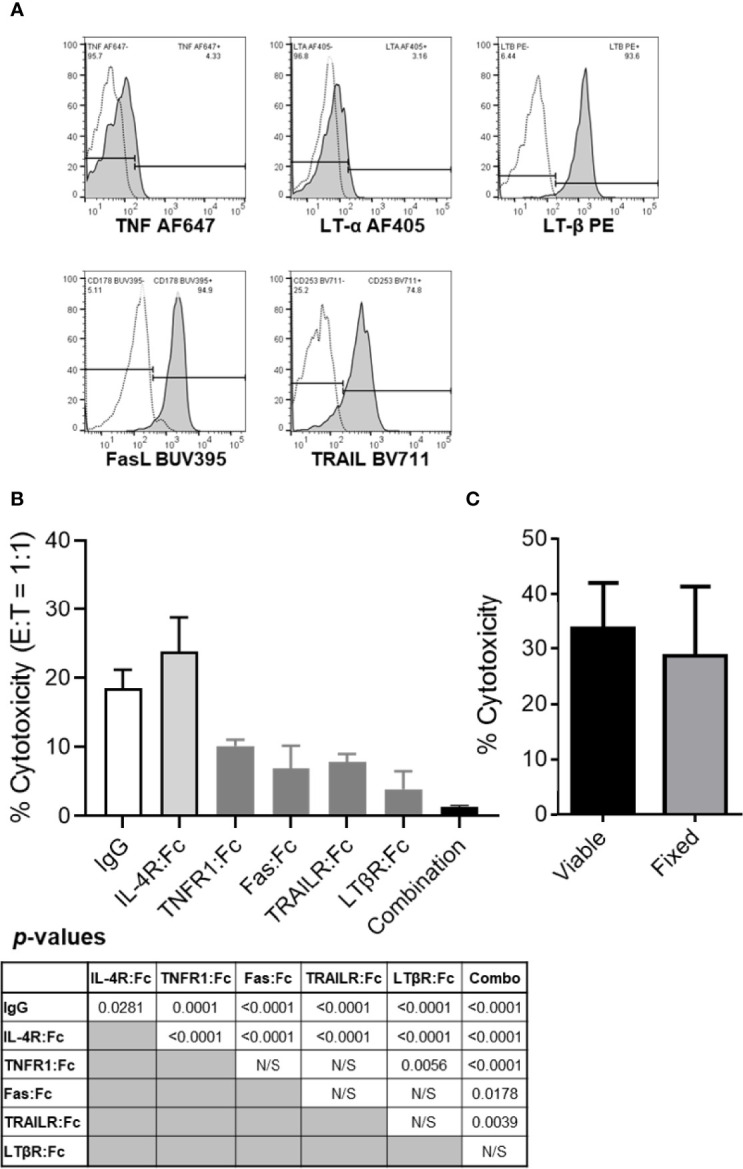
The tumor killing ability of resting B cells is inhibited by TNF, FasL, TRAIL and/or LT-α1β2 blockades but not by cellular fixation with paraformaldehyde. (A) Healthy donor PBL were stained for flow cytometry per Materials and Methods. Lymphocytes were gated based on their FSC vs SSC profile and single-cell status and, subsequently, evaluated for CD3 and CD19 expression. CD19+CD3- B cells were analyzed for surface TNF, LT-α, LT-β, FasL and TRAIL expression levels. Representative data from one of the four healthy donors evaluated are shown. Empty histograms represent IgG controls, and filled histograms represent the TNFSF ligand staining. (B) TNFSF receptor-Fc fragment fusion proteins were used to block healthy donor B cell cytotoxicity of PCI-13 cells at 1:1 E:T ratio. Where indicated, effector cells were preincubated with 10 μg/mL of the individual or combined (combination) TNFSF receptor-Fc fusion proteins specific for TNF (TNFR1:Fc), FasL (Fas : Fc), TRAIL (TRAILR : Fc) and LT-α1β2 (LTβR:Fc) for 1h at 37°C. Controls included IgG1 isotype and IL-4R:Fc fusion protein controls. Following preincubation with antagonistic agents, the cytotoxic activity of B cells was assessed using the 3-h MTT assay. Data represent the mean % cytotoxicity of 6 replicates ± STDEV, and statistical significance was calculated using the one-way ANOVA. Calculated p-values are listed in the summary table below the figure. A representative of 3 experiments is shown. (C) Resting healthy donor B cells fixed with 1% paraformaldehyde were compared to their viable counterparts for their ability to kill PCI-13 cells. The cytotoxic activity of B cells was assessed using the 24-h MTT assay at 3:1 E:T ratio. Data represent the mean % cytotoxicity of 4 replicates ± STDEV, and statistical significance was calculated using the two-tailed Student’s t-test. A representative of 2 experiments is shown.
