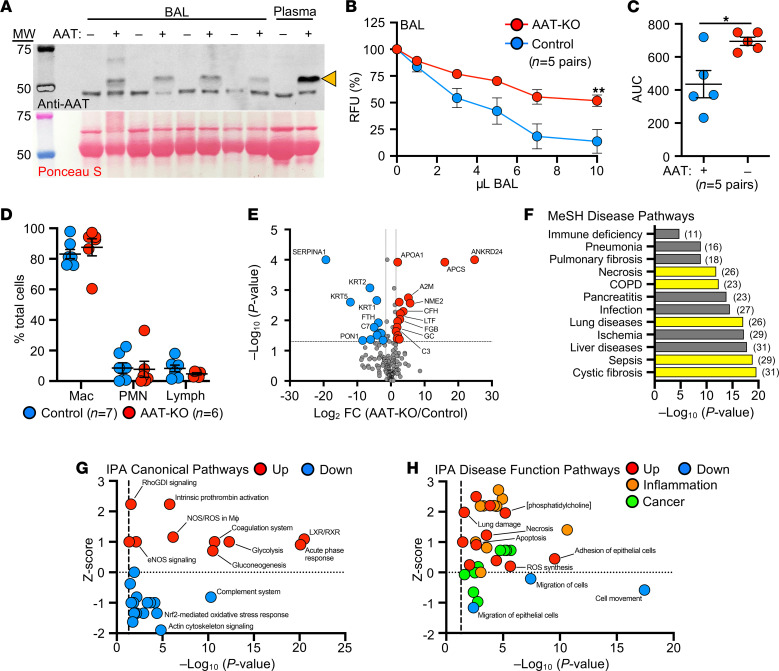
Figure 5. BAL from AAT-KO ferrets has reduced NE inhibitory capacity, and secreted proteome suggests enhanced inflammation and lung damage pathways.
(A) Western blot of BAL from AAT-KO (dash) and matched control ferrets (plus); arrowhead indicates AAT band in plasma as positive and negative controls. Ponceau-stained blot as loading control. (B) NE inhibitory capacity of increasing volumes of BAL from AAT-KO and matched control ferrets (n = 5 pairs; P value by mixed effects model, P = 0.005). (C) Quantification of NE inhibitory capacity by calculating the AUC in B (n = 5 pairs; P value by Student’s t test, P = 0.0297). (D) Differential of cell types in BAL from control and AAT-KO ferrets (n = 6–7 ferrets; P value by Student’s t test, P > 0.3 for each cell type). Mac, macrophages; PMN, polymorphonuclear leukocytes. (E) Volcano plot of more than 200 BAL proteins from 7 age-matched AAT-KO and control ferrets (red circles, upregulated; blue circles, downregulated; gray circles, indeterminate or not statistically significant; n = 7 matched pairs; P value by Scaffold software t test). (F) List of selected significant disease pathways discovered using MeSH analysis of the BAL proteome. Yellow bars denote disease-associated hits that are relevant to lung diseases, and numbers in parentheses indicate number of proteins found in the pathway. (G and H) IPA performed on proteomics data where (G) canonical and (H) disease function pathways are plotted against z score and –log10(P value). In both panels, red indicates upregulated and blue downregulated pathways by z score; in H inflammation-related pathways are in orange and cancer-related pathways are in green. Graphs in B–D show mean ± SEM. *P < 0.05, **P < 0.01. FC, fold change.