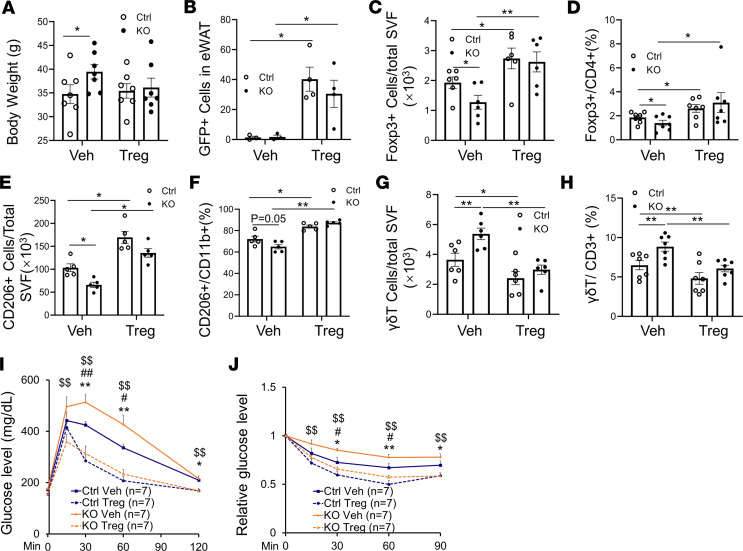
Figure 5. Adoptive transfer of Tregs reverses COX-2–KO–caused AT inflammation and insulin resistance.
For the following studies, mouse GFP+CD4+ T cells were isolated from lymph nodes and spleens of Foxp3-eGFP mice, and IP injection of CD4+GFP+ T cells which were positive Treg cells and CD4+GFP- as negative control cells to 8 weeks HFD-fed COX-2–KO and control (Ctrl) mice. (A) There was little effect of adoptive transfer on the BW in COX-2–KO and control mice 2 weeks post transfer. Flow cytometry analysis of CD4+GFP+ cells (B), CD4+Foxp3+ Treg cells (C) and the proportion of Foxp3+ Treg in CD4+ cells (D) in eWAT showed the successful transfer of Tregs in AT. Adoptive transfer of Tregs increased CD11b+CD206+ cell fraction (E) and the proportion of CD11b+CD206+ in CD11b+ cells (F), while suppressed γδT+CD3+ cell population (G) and the proportion of γδT+CD3+ cell in total CD3+ cells (H). Adoptive transfer of Treg cells rescued COX-2 deficiency-induced glucose (I) and insulin (J) intolerance. *P < 0.05 and **P < 0.01 Ctrl vehicle (Veh) vs. Ctrl Treg; #P < 0.05 and ##P < 0.01 for Ctrl Veh vs. KO Veh; $$P < 0.01 for KO Veh vs. KO Tregs; no significant difference was found between Ctrl Tregs and KO Tregs. (A–H) n = 4–7/group. (B) Representative data from 3 independent experiments are reported. ANOVA was used to analyze the data in this figure. Data are reported as mean ± SEM. *P < 0.05; **P < 0.01.