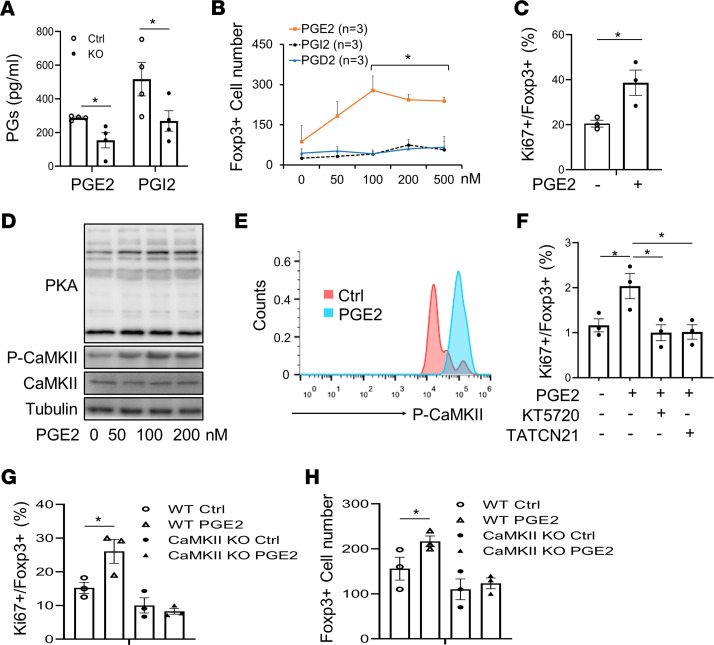
Figure 6. Adipocyte COX-2 promotes resident Treg proliferation through PGE2.
(A) COX-2 deficiency suppressed the secretion levels of PGE2 and PGI2 in primary adipocytes. COX-2–KO and control (Ctrl) primary adipocytes were changed to fresh medium and cultured for 2 hours. Medium was collected and used to determine the levels of PGE2 and PGI2, using an ELISA kit. (B, C, E, and F) AT Tregs were isolated from AT for these studies. Treatment of PGE2 but not PGD2 and PGI2 increased the population of Foxp3+ Tregs in a dose-dependent manner (B), and treatment of 100 nM PGE2 induced proliferation of Tregs as indicated by the staining of Ki67 (C). Intracellular Ki67+ and Foxp3+ Tregs were determined by flow cytometry analysis. AT Tregs were treated with DMSO, PGD2, PGE2, or PGI2, with indicated doses for 24 hours. *P < 0.05 compared with the group without treatment. (D) Treatment of PGE2 stimulated activation of PKA and CaMKII in differentiated Tregs in a dose-dependent manner. CD4+-naive T cells were isolated from a single-cell suspension from lymph nodes and spleens and then differentiated into CD4+Foxp3+ Tregs. Differentiated Tregs were treated, or not, with PGE2 for 1 hour. Representative data from 3 independent experiments are reported. (E) PGE2 treatment stimulated phosphorylation of CaMKII in AT Tregs. n = 3/group. (F) Inhibiting PKA by 5 μM KT 5720 or inhibiting CaMKII by 5 μM TATCN21 suppressed PGE2-treatment–induced proliferation of AT Tregs. AT Tregs were treated with KT 5720 or TATCN21 for 1 hour, followed by co-treatment with PGE2 for 24 hours. n = 3/group. CaMKIIγ deficiency blocked PGE2-stimulated CD4+Foxp3+ Treg proliferation, indicated by Ki67 expression (G) and total Treg fraction (H). Primary Tregs were isolated from AT of CaMKIIγ-KO and WT mice and treated with 100 nM PGE2 for 24 hours, followed by flow cytometry analysis. (A–C) The t test was used for data analysis. (F–H) ANOVA was used for data analysis. Data are reported as mean ± SEM. *P < 0.05; **P < 0.01.