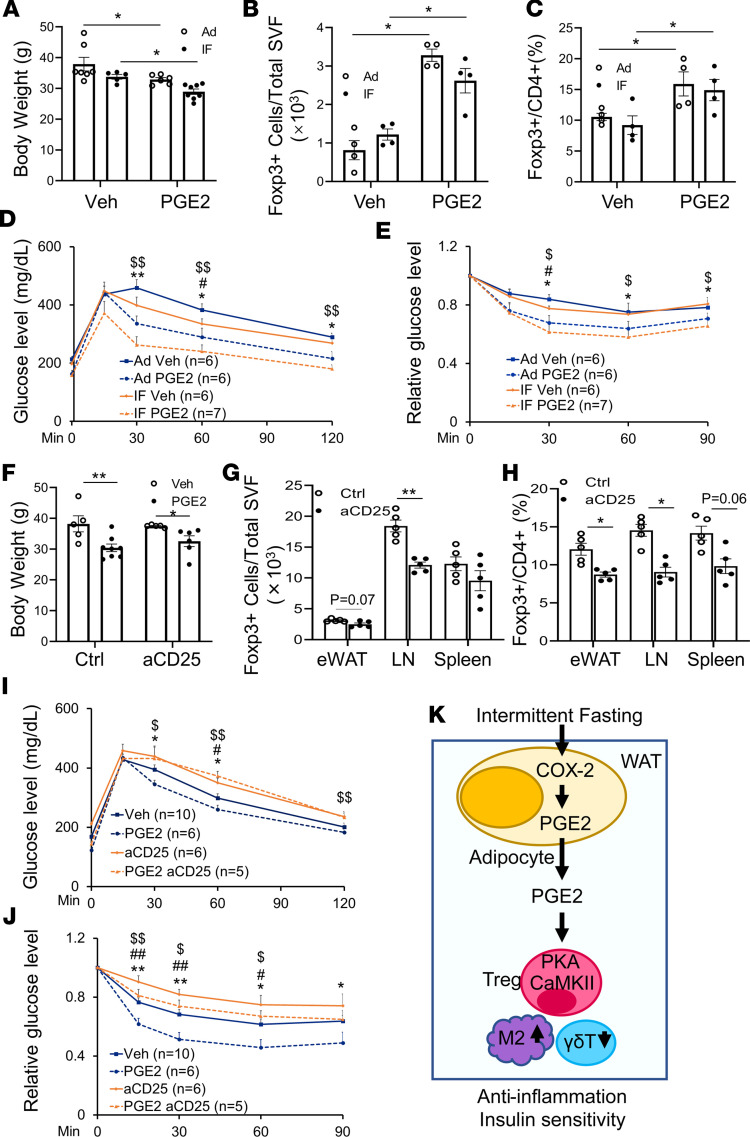
Figure 7. The PGE2/Treg axis is indispensable for the antiinflammatory and insulin-sensitizing effects of IF.
HFD-fed COX-2–KO mice were fed on an IF schedule or Ad for 4 weeks. Two weeks after IF, mice were injected with PGE2 or vehicle (Veh) for 2 weeks. (A) PGE2 administration resulted in significantly decreased body mass of COX-2–KO mice under both IF and Ad conditions. n = 5–8/group. Treatment with PGE2 restored the AT Treg population (B) and the proportion of Tregs in CD4+ cells (C) in COX-2–KO mice under both IF and Ad conditions. n = 4/group. PGE2 administration improved glucose (D) and insulin (E) tolerance in COX-2–KO mice under both IF and Ad conditions. *P < 0.05 and **P < 0.01 for Veh vs. PGE2 with Ad diet; #P < 0.05 for Ad vs. IF with Veh treatment; $P < 0.05 and $$P < 0.01 for IF Veh vs. IF PGE2. HFD-fed C57BL/6 mice were administered CD25 neutralizing antibody for 2 days, followed by PGE2 injection. (F) Blocking the Treg pathway had no significant effect on the antiobesity effect of PGE2, as indicated by the body mass. n = 5–8/group. (G and H) Neutralization of CD25 diminished the inducing effects of PGE2 on the AT Treg population. n = 5/group. Blocking the Treg pathway impaired basal and PGE2-increased glucose (I) and insulin (J) tolerance. *P < 0.05 and **P < 0.01 for Veh vs. PGE2; #P < 0.05 and ##P < 0.01 for Ctrl vs. anti-CD25 (aCD25); $P < 0.05 and $$P < 0.01 for aCD25 vs. PGE2 aCD25. (K) Working model. ANOVA was used to analyze the data in this figure. All data are reported as mean ± SEM. LN, lymph node.