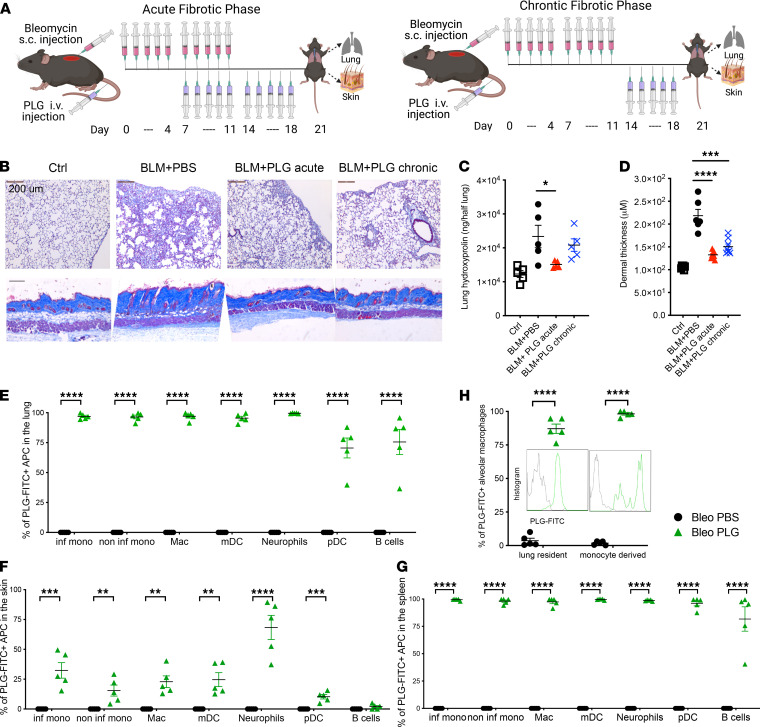
Figure 6. Therapeutic PLG nanoparticle treatment during the acute and chronic fibrotic phases attenuates pulmonary and dermal fibrosis.
(A) Schema of experimental setup and treatment regimens for acute and chronic phases of disease. (B) Representative images of Masson’s trichrome stain of the lung (top panels) and skin (bottom panels). Scale bar: 200 μm. (C) Lung hydroxyproline content and (D) dermal thickness (reported as mean ± SEM of 5 determinations per high-powered field from 5 mice per group). Percentage of PLG-FITC+ APCs in the lung (E), skin (F), and spleen (G) of control (Ctrl) and PLG-FITC+–injected mice. (H) Frequency and MFI of PLG-FITC+ alveolar macrophages (macs) in the lung. *P < 0.05; ***P < 0.001; ****P < 0.0001 via 1-way ANOVA followed by the Šidák’s multiple comparison test. inf mono, inflammatory monocyte; mDC, myeloid DC; noninf mono, noninflammatory monocyte; pDC, plasmacytoid DC.