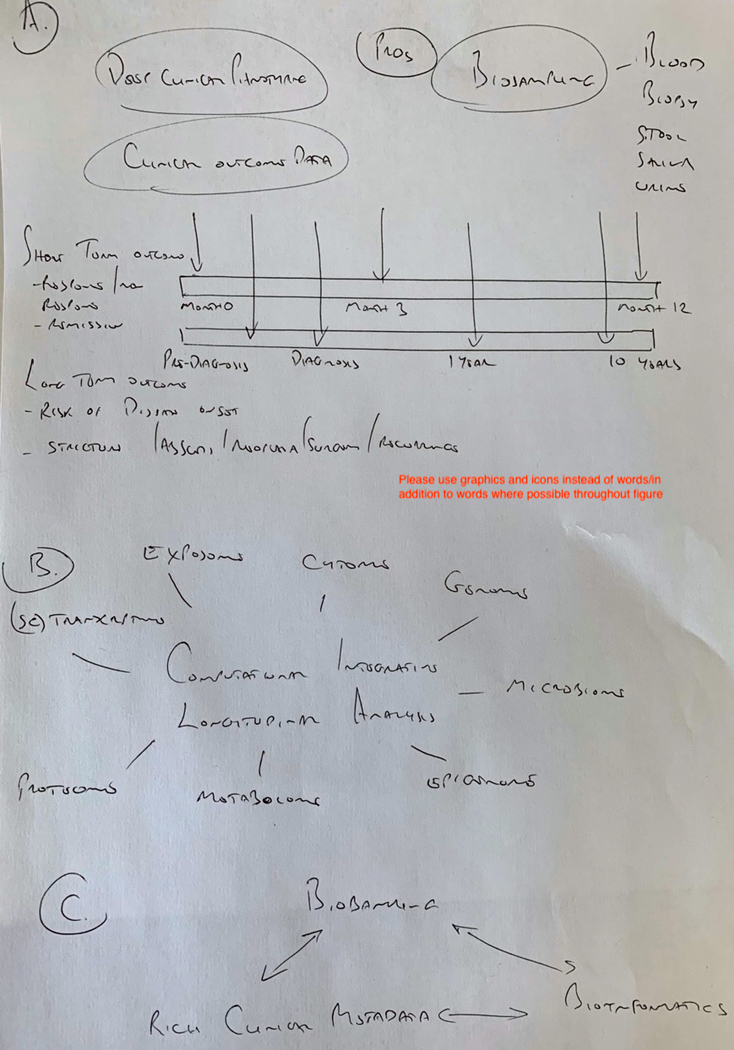
Figure 3:
Conceptual basis for optimal future precision medicine clinical trial and cohort design for short term and long-term outcomes in inflammatory bowel disease
A. Future observational cohort or interventional trials in precision medicine should be designed around relevant outcomes of interest. Short term outcomes such as treatment response/remission, non-response, loss of response and durable remission should have at least 1 year, ideally longer follow up. For Long term studies assessments should begin at diagnosis (or pre-diagnosis to assess disease risk or prevention) and continue for many years to study complications of disease such as stricture formation, abscess, neoplasia, surgery or postoperative recurrence. Crucial for both types of study is longitudinal collection of clinical outcome data, coupled with patient reported information, deep clinical phenotyping and appropriate biosampling to collect multiple tissue or sample types, at multiple timepoints. B. Biosamples should be analysed using multi-omic technologies or hypothesis-led targeted phenotypic assays and data integrated by computational means. This will provide multidimensional biological understanding, for example of disease progression, response to therapy, and biomarker discovery and validation. C. Remaining biosamples should be archived in biorepositories to facilitate targeted laboratory functional exploration or validation of biological insight informed by -omic approaches. Key to success is the association of these biorepositories with clinical metadata and bioinformatic pipelines to identify and refine big data discoveries and to formulate relevant hypotheses.