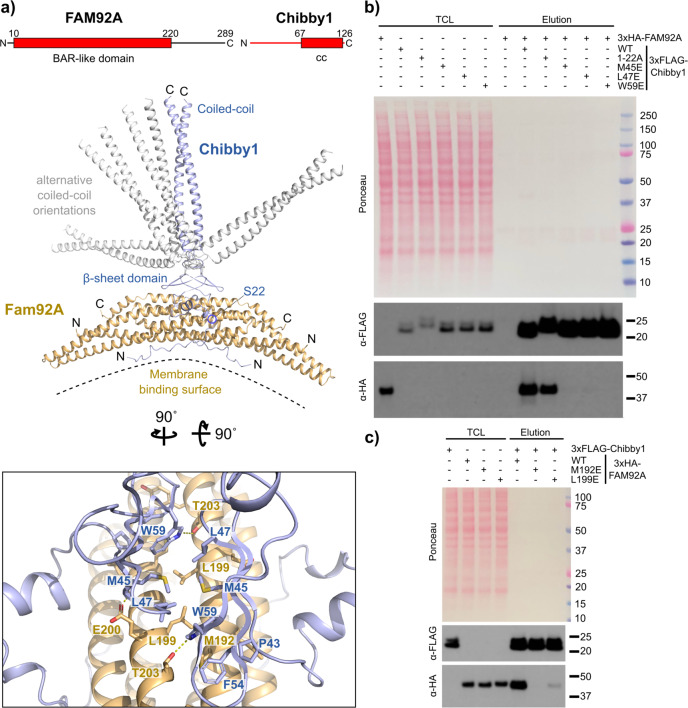
Fig. 3. AF2 predicts the structure of the FAM92A-Chibby1 complex.
a Ribbon representation of the structure of the human FAM92A-Chibby1 complex as predicted by AF2. The top five predictions were virtually identical in the FAM92A BAR domain and the Chibby1 beta-sheet domain and mainly differed in the orientation of Chibby1’s coiled-coil domain. The different coiled-coil orientations are shown for all five models (in blue or grey). Shown boxed is a detailed view on the interface between the beta-sheet domain of Chibby1 and the BAR domain of FAM92A. Selected sidechains are shown as sticks and are labelled. Dotted yellow lines designate hydrogen bonds. The rotation angles to obtain this view are indicated. In the domain organisation scheme of FAM92A and Chibby1, cc denotes a predicted coiled coil and amino acid residue numbers indicate domain boundaries. Coloured in red are the protein parts shown in the structural models. b The AF2-predicted interface between the Chibby1 beta-sheet domain and the FAM92A BAR domain is essential for complex formation. Western blot showing a pull-down experiment with lysates from cells expressing 3xHA-tagged FAM92A or the indicated 3xFLAG-tagged Chibby1 constructs. TCL: Total cell lysate. c As in panel b, but pulldown with 3xFLAG-tagged Chibby1 and the indicated 3xHA-tagged FAM92A constructs.