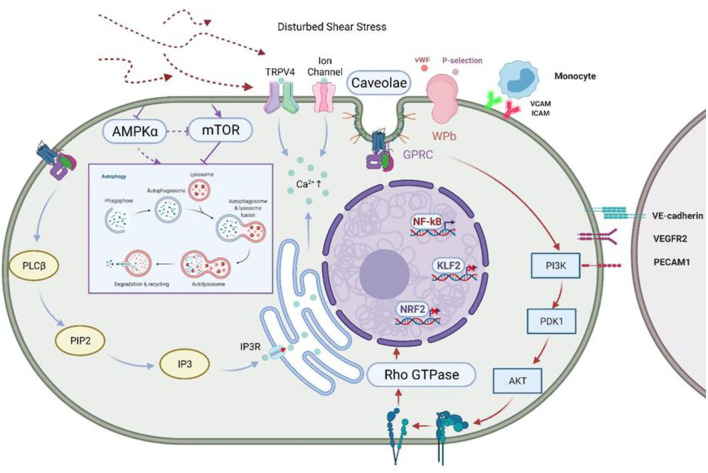
Figure 2.
Disturbed shear stress activates ECs and inhibits autophagy. Shear stress inhibits AMPKα and activates mTOR, two classical “autophagy switches,” to inhibit autophagy. Glycocalyxin acts as the first pass-through sensor in the face of shear stress, converting mechanical signals into physiological signals, which are then passed on through ion channels, caveolae, and integrins, amongst others (42). Shear stress regulates endothelial cell behavior through GPCR and the mechano-sensing complex formed by VE-cadherin and VEGFR-2/VEGFR3, which stimulate PI3K/AKT signaling and activate integrins in an “outside-in” manner, leading to the cis-activation of GTPases and the regulation of the expression of various transcription factors (28). This leads to the cis-activation of GTPases and promotes the expression of transcription factors. In addition, stimulated VE-cadherin and VEGFR2/VEGFR3 form a mechano-sensing complex that transmits signals to neighboring cells. At the same time, Ca2+ is stored in the endoplasmic reticulum via IP3R by the PLCβ/PIP2/IP3 signaling pathway in response to perturbed shear stress. Activation of non-selective cation channels such as TRPV4 and leads to the influx of more Ca2+ and hyperpolarizes the membrane. At the same time, shear stress regulates platelet behavior by inducing the release of P-selectin and vWF from WPBs (46). AMPK, AMP-activated protein kinase; mTOR, mammalian target of rapamycin; GPCR, G protein-coupled receptors; VEGFR, vascular endothelial growth factor receptor; PI3K, phosphatidyl inositol 3 kinase; IP3R, inositol trisphosphate receptor; PLCβ, Phospholipase C Beta 3; PIP2, phosphati-dylinositol-4,5-bisphosphate; IP3, inositol triphosphate; TRPV4, transient receptor potential; , Ca2+-sensitive K channels; WPBs, Weibel-Palade bodies; vWF, von Willebrand factor.