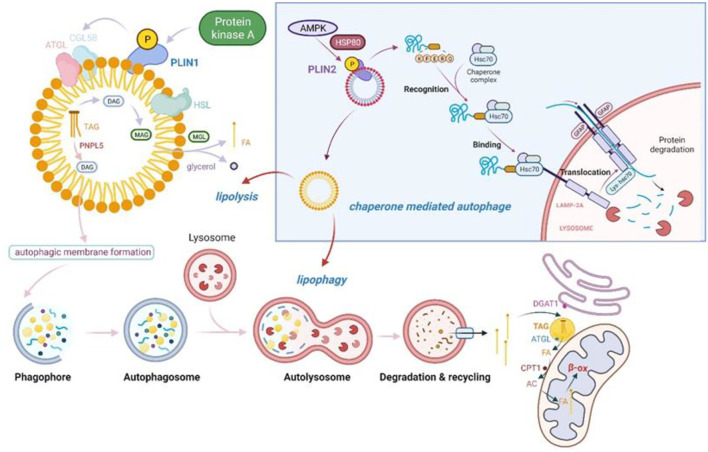
Figure 4.
Lipolysis and lipophagy in tandem with molecular chaperone-mediated autophagy (CMA). PKA phosphorylates PLIN1, a member of the PAT protein family, and causes its degradation. This induces the release of CGI-58, which then initiates TAG catabolism via activated ATGL. ATGL catalyzes the first step of TAG hydrolysis, producing DAG and free fatty acids. The second step of lipolysis is dependent on the activation of HSL, the rate-limiting enzyme in the lipolysis cascade reaction (113). HSL hydrolyses DAG to produce monoacylglycerols and FFAs, which are excreted from LDs and enter the mitochondria for β-oxidation (113). In addition, the novel neutral lipase PNPLA5 in LDs has triglyceride hydrolase activity and can hydrolyze TAG to DAG, promoting autophagic vesicle membrane formation in a PNPLA5-dependent manner. Furthermore, LDs are also present as a 'staging area' for the entry of FFAs into mitochondria for β-oxidation, thus protecting them from damage by toxic ACs and excess FFAs (114). CMA may be an important link in the initiation of lipophagy and lipolysis. AMPK phosphorylates PLIN2 on LDs in an HSP80-dependent manner, allowing it to be degraded by lysosomes by way of CMA. The degradation of PLIN2 allows lipid droplets to undergo subsequent lipophagy and lipolysis. PKA, protein kinase A; PLIN, perilipin; CGI-58, comparative gene identification-58; TAG, triacylglycerides; ATGL, adipose triglyceride lipase; DAG, diacylglycerol; HSL, hormone-sensitive lipase; FFAs, free fatty acids; LDs, lipid droplets; PNPLA5, Patatin-like phospholipase domain containing 5; DGAT1, diacylglycerol acyltransferase 1; CPT1, carnitine palmitoyltransferase 1; AC, acylcarnitine.