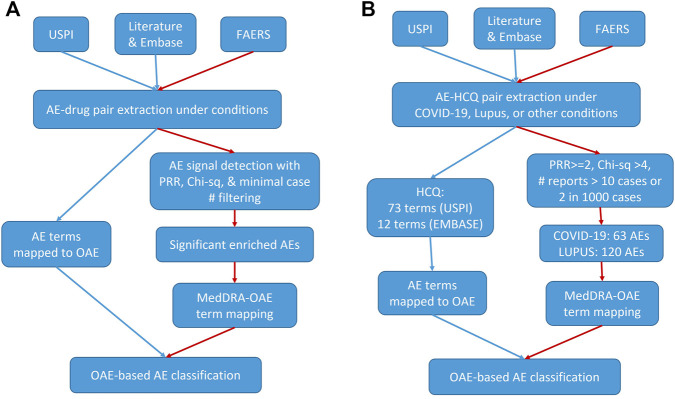
FIGURE 1.
Workflow for our drug AE analysis. (A) General workflow. (B) HCQ specific workflow. The red paths presented with red edges represent the workflow of FAERS-specific drug AE analyses. USPI, United States Prescribing Information. FAERS, FDA Adverse Event Reporting System. AE, Adverse Event. PRR, Proportional Reporting Ratio. OAE, Ontology of Adverse Event. HCQ, hydroxychloroquine.