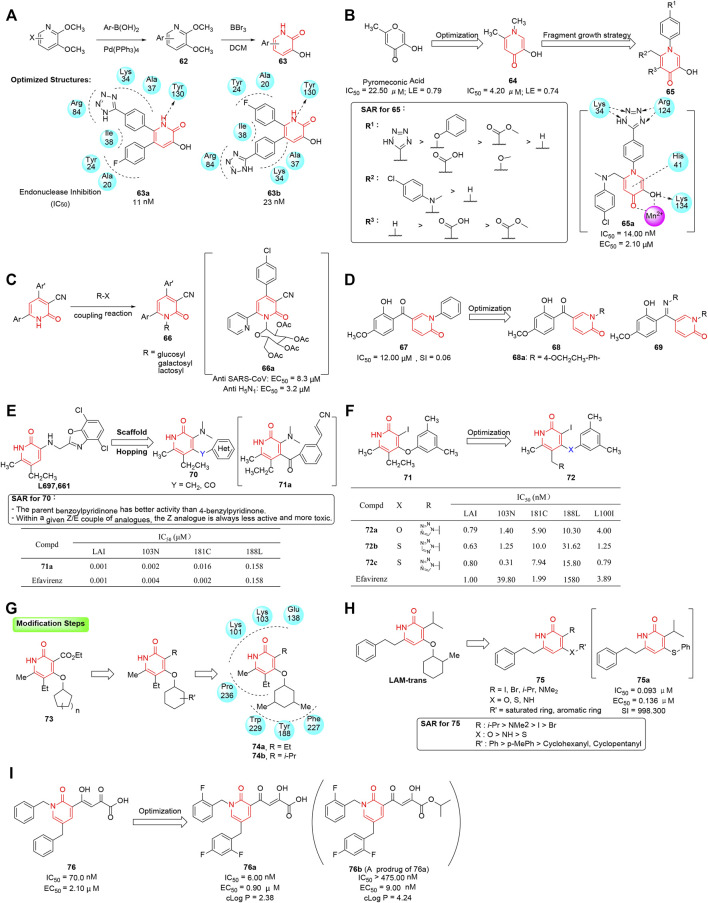
FIGURE 8.
(A) Synthesis of phenyl substituted 3-hydroxypyridin-2(1H)-ones as endonuclease inhibitors. (B) From hit to lead for inhibitors of influenza PA endonuclease. (C) Antiviral activity of 2-ONN-based nucleoside analogs. (D) 2-pyridinone derivatives as inhibitors against HBV DNA replication and hepatitis B e-antigen (HBeAg) separately. (E) Design of pyridin-2(1H)-ones as NNRTIs by using a scaffold hopping approach (Benjahad et al., 2004). (F) Biological activity of IOPY and ISPY analogs (Benjahad et al., 2007). (G) Optimization of pyridinone-based NNRTIs. (H) SAR of pyridin-2(1H)-ones as inhibitors of HIV reverse transcriptase. (I) Optimization of 1,5-dibenzyl-2-pyridinones as InSTIs.