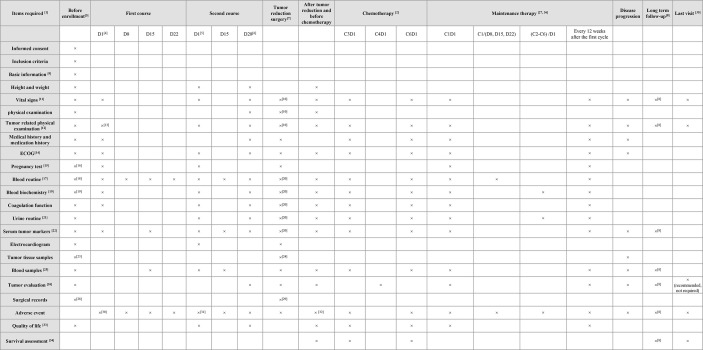
Figure 2.
Follow-up plan. 1In addition to the prescribed visit schedule, local researchers may conduct more frequent inspections as per patients’ requirements, which may include blood and urine routine examination, blood biochemistry, ECG, CT, and serum tumor biomarker (within one week); 2Refers to platinum-based chemotherapy post-surgery and included information collected from the patient’s medical record; 3It should not be completed >4 weeks before enrolment, excluding exceptional cases; 4Required to be completed within 72 hours of the start of the cycle 1 ( ± 1D); 5Required to be completed within 72 hours of the start of the cycle 2 ( ± 1D); 6Required to be completed within 72 hours from the beginning of cycle 2 treatment D28 (± 1D); 7Required to be completed within 3 days pre-operation; 8Once every 12 weeks after maintenance treatment and once every 24 weeks after two years; 9Includes name, age, gender, place of origin, contact details and date of admission; 10Time interval from last inspection should be >7 days, otherwise the inspection shall be canceled; 11Includes heart rate, blood pressure, pulse and respiration; 12Records the tumor size at least; 13Recommended, non-mandatory; 14The United States Eastern Cooperative Oncology Group (ECOG) physical status scores; 15Blood or urine β-HCG test; 16Completed within two weeks before enrollment; 17Includes neutrophil/platelet count, and Hb level; 18Atleast 7 days before enrollment; 19Includes measurement of serum creatinine and electrolytes, total bilirubin, ALT/AST; 20Time interval from the last inspection should not be <1 week, otherwise the inspection shall be canceled; 21Includes measurement of creatinine, urea nitrogen, and erythrocytes; 22Includes testing of CA125, CA199, CEA, and HE4 related markers; 23Biopsy done by laparoscopy, laparotomy, or coarse-needle aspiration; 24Primary and metastatic tumor tissues obtained by laparotomy/laparoscopic tumor reduction surgery; 25Samples in 2ml EDTA anticoagulant tube were used for HRD detection before enrollment and after two courses of treatment. Samples of 10ml Streck tube were collected before enrollment, D15 and D28 of the first course of chemotherapy, D15 of the second course of chemotherapy, and before tumor reduction surgery and subsequent therapy; 26Abdominal CT or MRI is recommended for evaluation instead of ultrasound alone; 27If blood routine tests are abnormal during maintenance treatment, then it should be carried out every 3 days and closely monitored until it becomes normal; 28The operation record of the biopsy should be sent to the research center for record within 7 days; 29Surgical records should be completed within 24 hours after the surgery and sent to the research center within 7 days for reference; 30All adverse events to be documented from first day of receiving niraparib to post 30 days of treatment termination; 31All adverse events to be documented from first day of receiving niraparib to post 30 days of treatment termination; 32Postoperative adverse events (D1 to D28); 33Includes FACT-O, HADS, ISI, IPAQ, and EQ-VAS; 34Recurrence and time of recurrence, death and time of death, whether to continue follow-up and last follow-up time are recorded; 35The last follow-up before withdrawal; 36After the completion of first-line chemotherapy, patients will receive maintenance treatment with niraparib within 12 weeks. ALT/AST, alanine aminotransferase/aspartate aminotransferase; CA, cancer antigen; CEA, carcinoembryonic antigen; CT, computed tomography; D, day; ECOG, Eastern Cooperative Oncology Group; ECG, electrocardiogram; EDTA, ethylenediaminetetraacetic acid; EQ-VAS, EuroQol-visual analog scales; FACT-O, Functional Assessment of Cancer Therapy-Ovarian; HADS, Hospital Anxiety and Depression Scale; Hb, hemoglobin; β-HCG, β-human chorionic gonadotropin; HE4, human epididymis protein 4; HRD, homologous recombination deficiency; IPAQ, International Physical Activity Questionnaire; ISI, Insomnia Severity Index; MRI, magnetic resonance imaging.