Fig 1. FAK activation and role in cardiomyocytes.
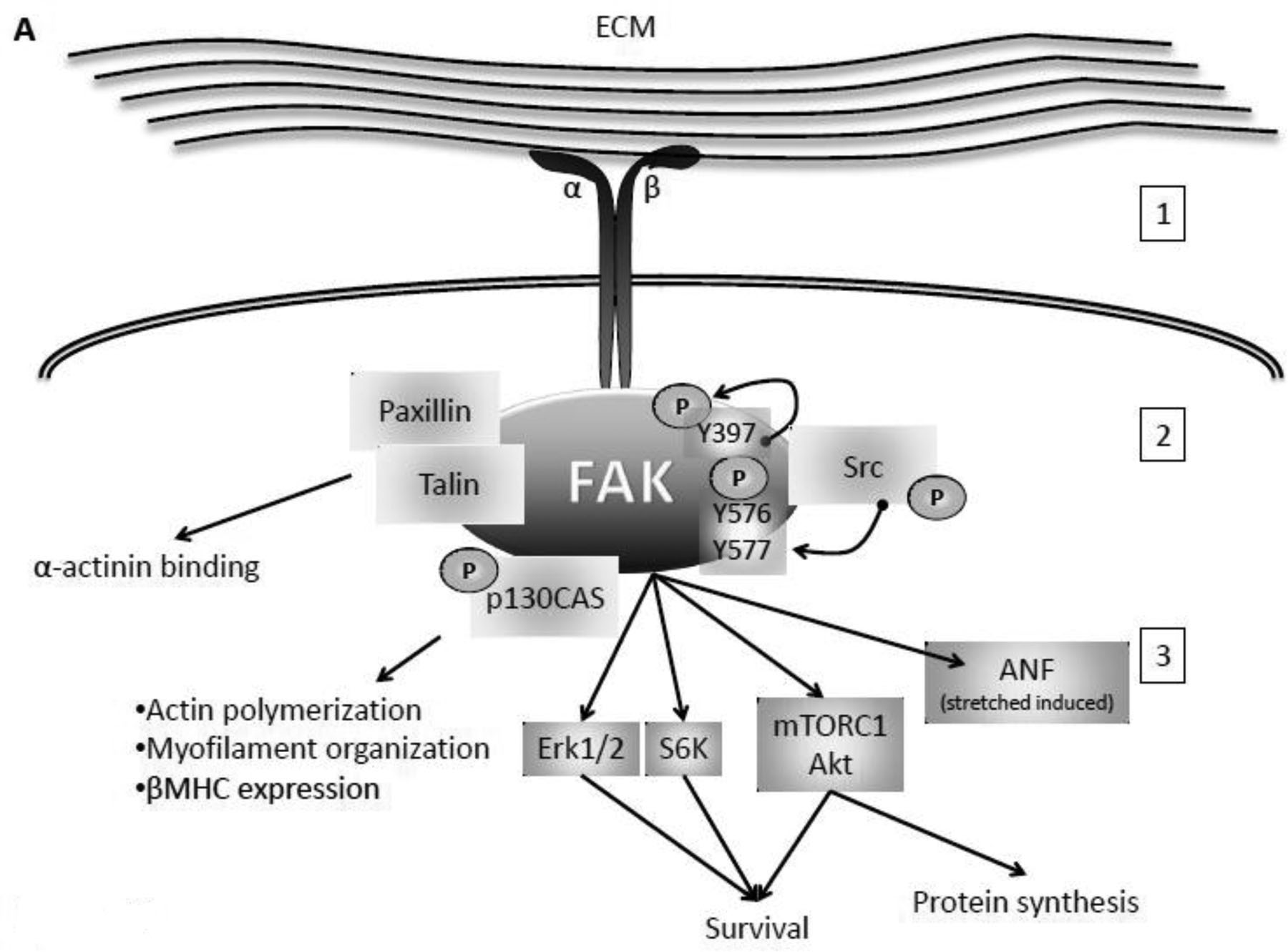
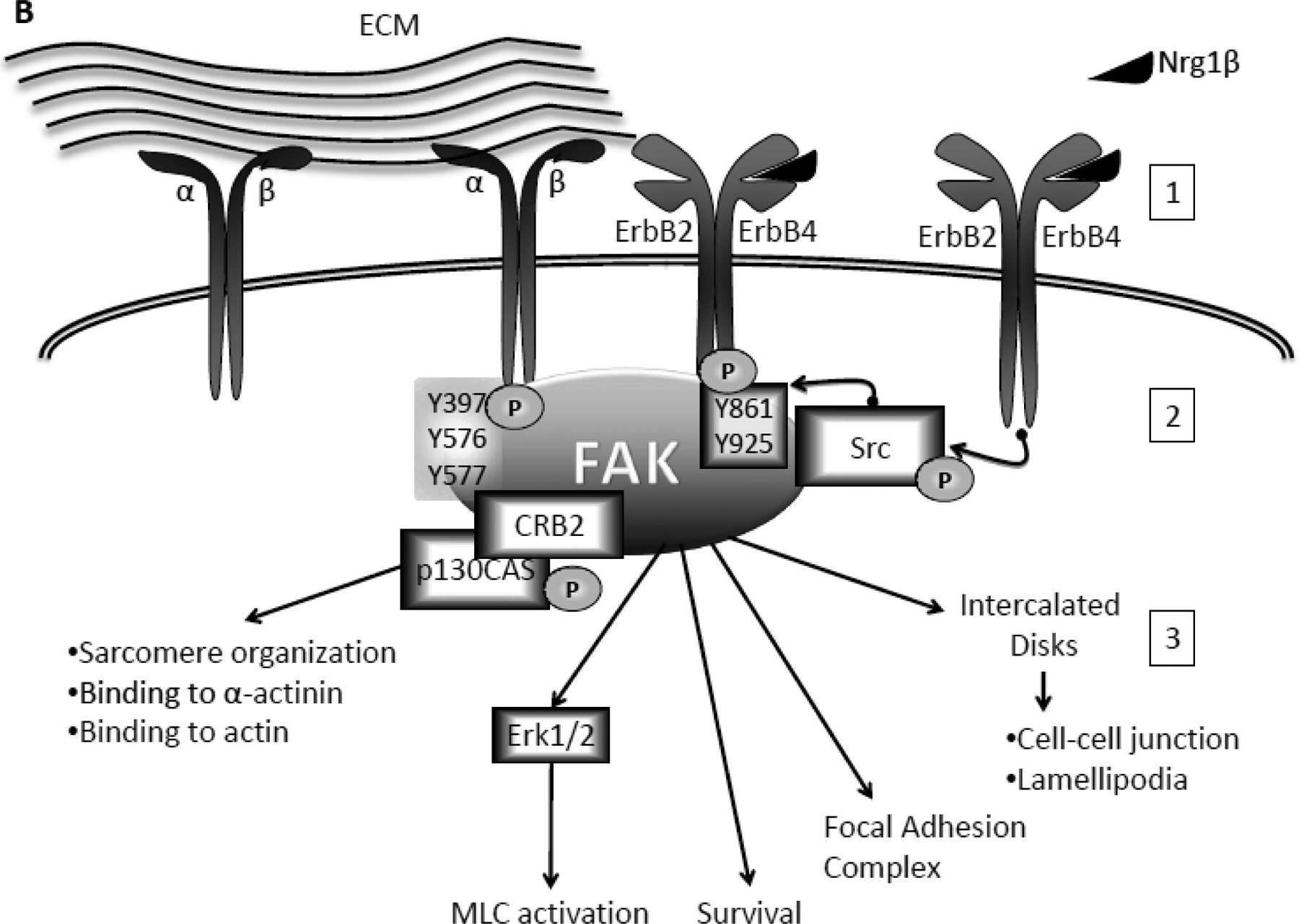
A. Integrin-dependent activation of FAK. Binding to the extracellular matrix (ECM) and mechanical stretch activates integrin receptors at the cell surface (1). To initiate intracellular signaling integrin dimer induces conformational changes in FAK and auto-phosphorylation on tyrosine (Y) 397. Src can then phosphorylate FAK at Y576 and Y577 in the activation loop (2). Activated FAK can then: interact with Paxillin and Talin to bind α-actinin; induce actin polymerization, myofilament organization, and expression of Myosine Heavy Chain (MHC) via p130CAS; and promote survival via Erk1/2, S6K, mTORC1, and Akt, protein synthesis via mTORC1 and Akt, and stretch induced expression of ANF (3). B. Nrg1β-specific phosphorylation of FAK and its role in cardiomyocytes. Upon binding to Nrg1β (1), the ErbB2/ErbB4 heterodimer induces phosphorylation of FAK at Y861 and Y925 via Src (2). Phosphorylated FAK: is involved in sarcomere organization and binding to actin and α-actinin via interaction with p130CAS and CRB adaptor proteins; induces activation of Myosin Light Chain (MLC) via Erk1/2; promotes myocyte survival and focal adhesion complex formation; and migrates to the intercalated disks where it promotes cell-cell interaction and lamellipodia formation (3).
