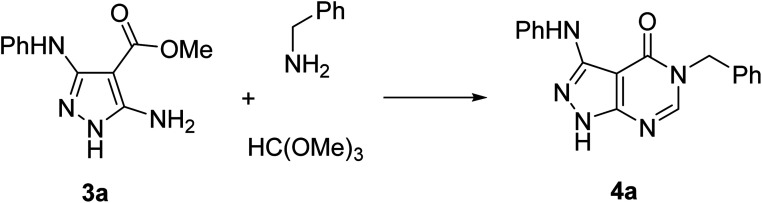
Optimisation of reaction conditions for the synthesis of 5-benzyl-3-phenylaminopyrazolo[3,4-d]pyrimidin-4-one (4a) under microwave irradiationa.
| ||||
|---|---|---|---|---|
| Entry | Solvent | Temp (°C) | Reaction time (min) | Yieldb (%) |
| 1 | Toluene | 160 | 35 | 10 |
| 2 | MeCN | 160 | 35 | 66 |
| 3 | EtOH | 160 | 35 | 72 |
| 4 | n PrOH | 160 | 35 | 35 |
| 5 | i PrOH | 160 | 35 | 53 |
| 6 | Eucalyptol | 160 | 35 | 28 |
| 7 | 2-MeTHF | 160 | 35 | 12 |
| 8 | EtOH | 160 | 45 | 75 |
| 9 | EtOH | 160 | 55 | 83 |
| 10 | EtOH | 160 | 65 | 75 |
| 11 | EtOH | 150 | 55 | 45 |
| 12c | EtOH | Reflux | 4320 | Tracesd |
| 13e | EtOH | 160 | 55 | 27 |
The reactions were performed in a Discover SP (CEM, USA) using 3a (1 mmol), trimethyl orthoformate (3 mmol), and benzylamine (3 mmol) in 2 mL of a solvent under a maximal microwave irradiation power of 150 W.
Isolated yield calculated on the basis of 3a.
The reaction was performed using conventional heating under reflux.
The traces are identified in the 1H NMR spectrum of the crude reaction mixture.
The reaction was performed using conventional heating in a Monowave 50 (Anton Paar, Austria).
