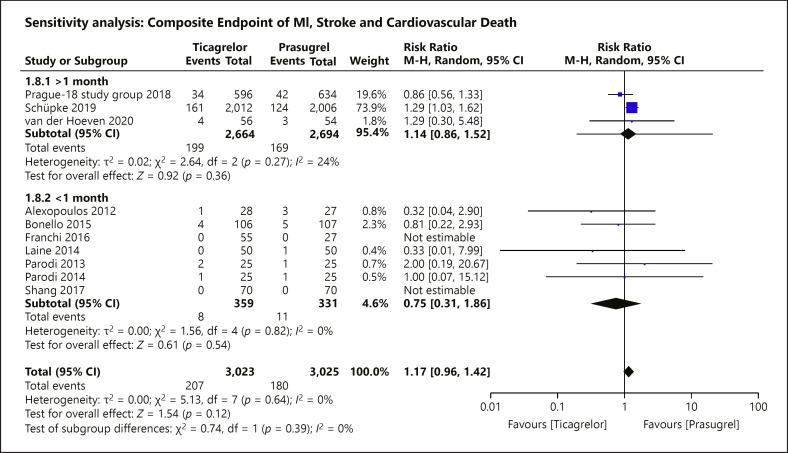
Fig. 8.
Sensitivity analysis of the primary composite endpoint stratified by length of follow-up. A prespecified sensitivity analysis stratifying the trials by the duration of follow-up was assessed for the primary composite outcome. There was no risk difference between the use of prasugrel and ticagrelor among all of the 3 subgroups, respectively (>1 month: RR = 1.14; 95% CI = 0.86–1.52; p = 0.36, I2 = 24%; =1 month: RR = 0.81; 95% CI = 0.22–2.93; p = 0.74; <1 month: RR = 0.71; 95% CI = 0.20–2.51; p = 0.59, I2 = 0%).