| ||||||
|---|---|---|---|---|---|---|
| ||||||
| Prod. | X | R1 | Yield | t (min) | TON | TOF |
| 9a | I | NH2 | 85 | 120 | 180 | 90 |
| 9a | Cl | NH2 | 65 | 150 | 138 | 39 |
| 9b | I | OMe | 92 | 70 | 195 | 168 |
| 9b | Br | OMe | 90 | 110 | 191 | 104 |
| 9c | I | Me | 85 | 90 | 180 | 120 |
| 9c | Br | Me | 75 | 120 | 159 | 79 |
| 9c | Cl | Me | 65 | 150 | 138 | 55 |
| 9d | I | H | 95 | 15 | 202 | 808 |
| 9d | Br | H | 95 | 20 | 202 | 612 |
| 9d | Cl | H | 92 | 100 | 195 | 117 |
| 9e | I | CHO | 93 | 60 | 197 | 197 |
| 9e | Br | CHO | 85 | 85 | 180 | 128 |
| 9e | Cl | CHO | 75 | 100 | 195 | 96 |
| 9f | Br | COMe | 95 | 90 | 202 | 134 |
| 9g | I | NO2 | 94 | 25 | 200 | 487 |
| 9g | Br | NO2 | 90 | 35 | 191 | 330 |
| 9g | Cl | NO2 | 87 | 55 | 155 | 155 |
| 9h | Br | CN | 80 | 90 | 170 | 113 |
| 9h | Cl | CN | 60 | 180 | 148 | 49 |
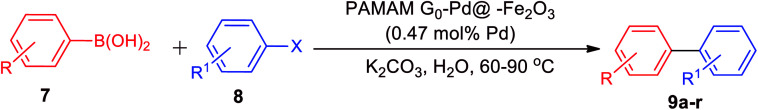
Reaction conditions: phenylboronic acid (1.2 mmol), aryl halide (1.00 mmol), K2CO3 (Et3N when we used the aryl chlorides) (2.00 mmol), catalyst (0.007 g, 0.47 mol% of Pd), H2O (2.0 mL), 60 °C (90 °C when we used the aryl chlorides); all yields are isolated.
