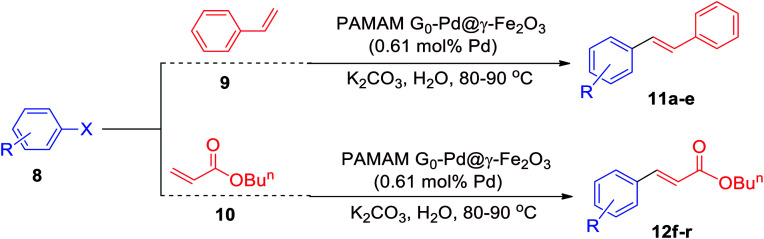
Mizoroki–Heck cross-coupling reactions of different aryl halides with styrene catalyzed by PAMAM G0-Pd@γ-Fe2O3a.
| ||||||
|---|---|---|---|---|---|---|
| ||||||
| Prod. | X | R | Yield | t (min) | TON | TOF |
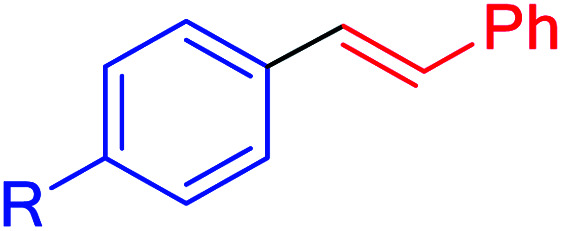
| 12a | I | Me | 75 | 180 | 122 | 41 |
| 12a | Br | Me | 65 | 180 | 106 | 35 |
| 12a | Cl | Me | 55 | 220 | 90 | 24 |
| 12b | I | OMe | 90 | 90 | 148 | 98 |
| 12c | I | H | 95 | 60 | 155 | 150 |
| 12c | Br | H | 95 | 80 | 155 | 117 |
| 12c | Cl | H | 90 | 120 | 147 | 73 |
| 12d | I | CN | 85 | 60 | 139 | 139 |
| 12e | I | NO2 | 95 | 45 | 155 | 206 |
| 12e | Br | NO2 | 95 | 50 | 155 | 186 |
| 12e | Cl | NO2 | 90 | 60 | 147 | 147 |
| ||||||
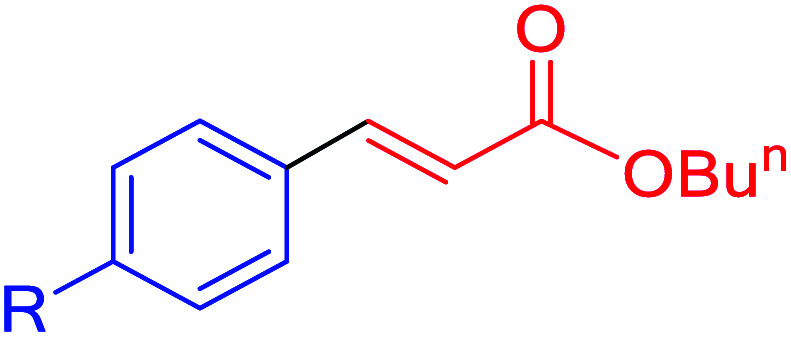
| 12f | I | OH | 90 | 120 | 147 | 73 |
| 12g | I | NH2 | 90 | 120 | 147 | 73 |
| 12h | I | OMe | 92 | 500 | 150 | 181 |
| 12h | Cl | OMe | 65 | 300 | 106 | 21 |
| 12i | I | Me | 80 | 120 | 131 | 65 |
| 12i | Br | Me | 80 | 140 | 131 | 56 |
| 12i | Cl | Me | 70 | 150 | 114 | 45 |
| 12j | I | H | 95 | 50 | 155 | 187 |
| 12j | Br | H | 90 | 75 | 147 | 117 |
| 12j | Cl | H | 80 | 100 | 131 | 78 |
| 12k | I | Cl | 85 | 55 | 139 | 153 |
| 12k | Br | Cl | 70 | 60 | 114 | 114 |
| 12l | I | Br | 75 | 70 | 122 | 105 |
| 12m | I | CO2Et | 95 | 40 | 131 | 65 |
| 12m | Br | CO2Et | 93 | 50 | 152 | 183 |
| 12m | Cl | CO2Et | 90 | 80 | 147 | 110 |
| 12n | I | COMe | 92 | 70 | 150 | 129 |
| 12o | Br | CN | 92 | 50 | 150 | 181 |
| 12o | Cl | CN | 85 | 120 | 139 | 69 |
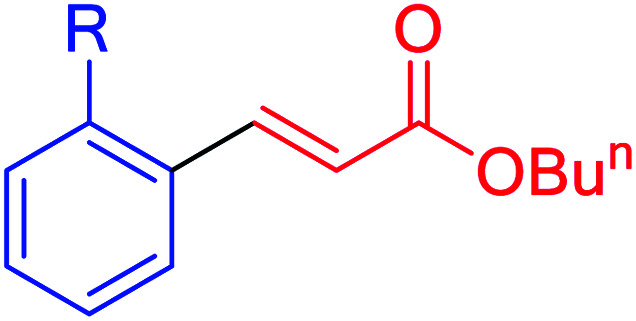
| 12p | I | NO2 | 95 | 30 | 155 | 77 |
| ||||||
| 12q | Br | Me | 65 | 130 | 106 | 49 |
| 12q | Cl | Me | 50 | 240 | 81 | 20 |
| 12r | I | NO2 | 92 | 70 | 150 | 129 |
Reaction conditions: styrene or n-butyl acrylate (1.5 mmol), aryl halide (1.00 mmol), K2CO3 (Et3N when we used the aryl chlorides) (2.00 mmol), catalyst (0.009 g, 0.61 mol% of Pd), H2O (2.00 mL), 80 °C (90 °C when we used aryl chlorides); all yields are isolated.
