Fig. 3: The known unknowns and potential pitfalls of retrospective biomarker analyses.
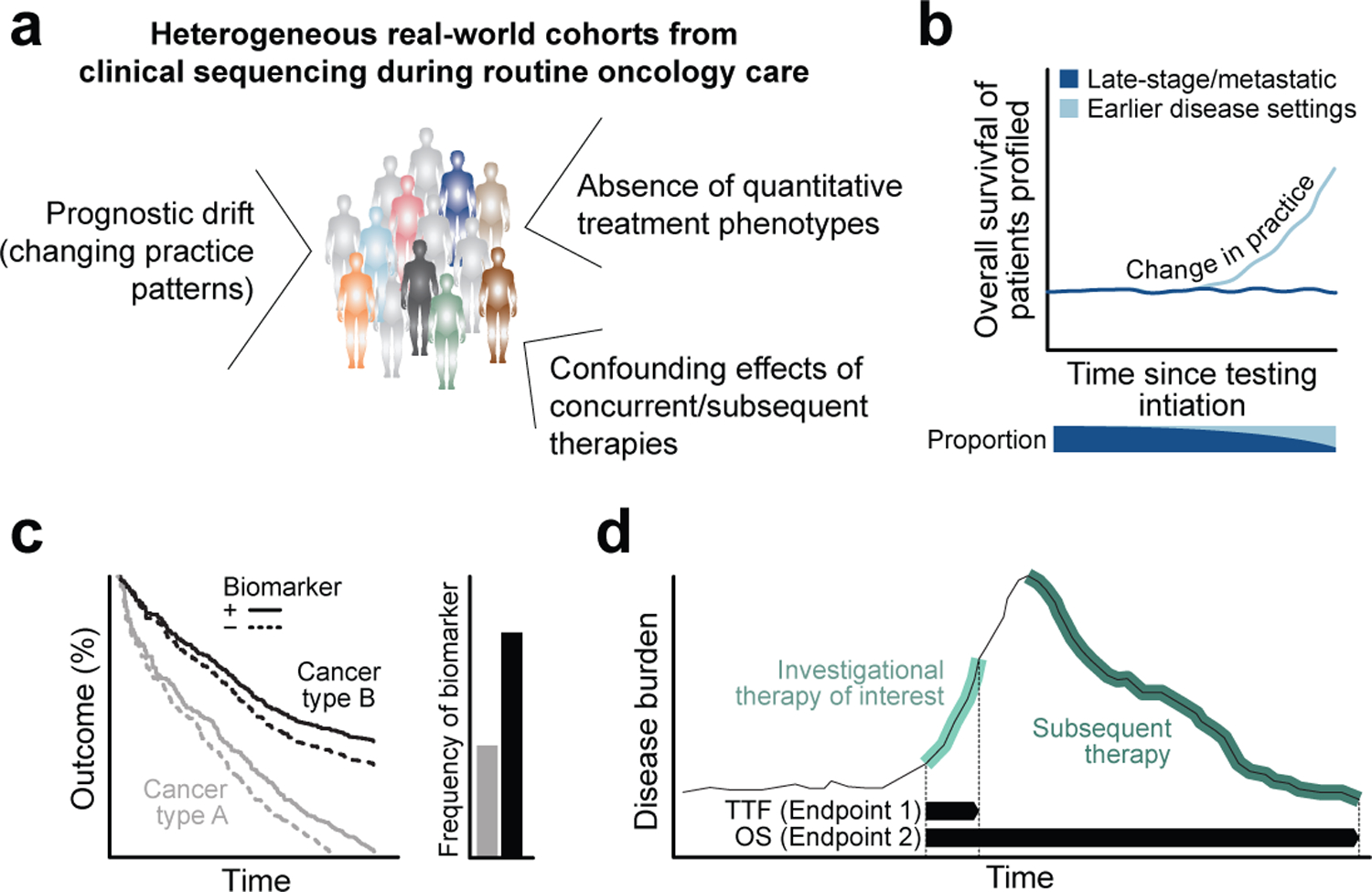
a) Various potential confounding factors that complicate the current generation of rigorous retrospective biomarker analyses using real-world clinical sequencing data in oncology. b) Over time, as clinical practice patterns change and the patient population for which clinical sequencing is routinely performed expands beyond late-stage and treatment refractory disease, the prognostic composition of the cohort will shift, leading to potentially spurious associations with outcome. c) Biomarker analysis pan-cancer can fail to discriminate between survival differences driven by underlying biology versus therapeutic intervention when affected cancer types have very different natural histories or the biomarker itself bestows favorable or worse prognosis. d) The effect of subsequent lines of therapy can confound key clinical endpoints in clinical sequencing cohorts composed of patients with heterogeneously administered therapies. TTF, time to treatment failure; OS, overall survival.
