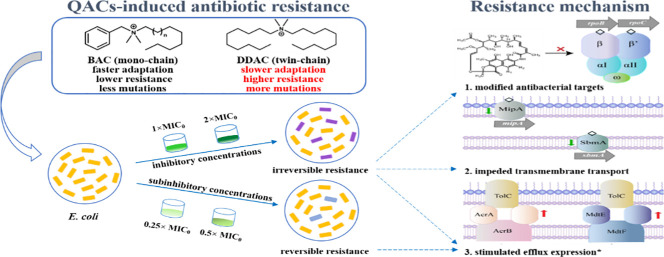
Abstract
The usage of quaternary ammonium compounds (QACs) as disinfectants has increased dramatically since the outbreak of COVID-19 pandemic, leading to potentially accelerated emergence of antibiotic resistance. Long-term exposure to subinhibitory level QACs can lead to multidrug resistance, but the contribution of mutagenesis to resistance evolution is obscure. In this study, we subcultured E. coli K-12 under subinhibitory (0.25 × and 0.5 × Minimum Inhibitory Concentration, MIC) or inhibitory (1 × and 2 × MIC) concentrations of benzalkonium chloride (BAC, mono-chained) or didecyldimethylammonium chloride (DDAC, twin-chained) for 60 days. The sensitivity of QAC-adapted cells to five typical antibiotics decreased significantly, and in particular, the MIC of rifampicin increased by 85 times. E. coli adapted faster to BAC but developed 20–167% higher antibiotic resistance with 56% more mutations under DDAC exposure. The broader mutations induced by QACs, including negative regulators (acrR, marR, soxR, and crp), outer membrane proteins and transporters (mipA and sbmA), and RNA polymerase (rpoB and rpoC), potentially contributed to the high multi-drug resistance. After QACs stresses were removed, the phenotypic resistance induced by subinhibitory concentrations of QACs was reversible, whereas that induced by inhibitory concentrations of QACs was irreversible. The different patterns and molecular mechanism of antibiotic resistance induced by BAC and DDAC is informative to estimating the risks of broader QACs present at varied concentrations in the environment.
Keywords: Benzalkonium chloride, Didecyldimethylammonium chloride, Antibiotic resistance, Whole-genome sequencing, Mutation
Graphical abstract
1. Introduction
Antimicrobial resistance (AMR) poses serious threats to public health globally. The abuse of non-antibiotic chemicals greatly promotes the emergence and spread of AMR (Baker-Austin et al., 2006; Li et al., 2016; Wang et al., 2020). Since the coronavirus pandemic in 2020, the use of disinfectants (e.g., surface cleaners, sanitizers) has increased dramatically across the world, which is expected to increase AMR in pathogenic microbes in the coming years (Mahoney et al., 2021). Among the 597 products listed in the U.S. Environmental Protection Agency's (EPA) List N: Disinfectants for Use Against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), 277 contain quaternary ammonium compounds (QACs) as active ingredients (United States Environmental Protection Agency, 2022). QACs are a group of cationic surfactants widely used as broad-spectrum antimicrobial agents in medical, industrial and household applications, which interact with negatively charged cell membranes to cause destruction and leakage of cell contents (Jennings et al., 2015; Tandukar et al., 2013). QACs environmental concentrations typically range from less than 1 μg/L to 2.8 mg/L, while the surge of their usage during the COVID-19 pandemic has resulted in significant increases in untreated wastewater (from 478 g day−1 to 2061 g day−1, +331%), residential dust (from 36.3 μg/g to 58.9 μg/g, +62%) and other environments (Clara et al., 2007; Ferrer and Furlong, 2001; Hora et al., 2020; Kreuzinger et al., 2007; Li and Brownawell, 2010; Martinez-Carballo et al., 2007; Alygizakis et al., 2021; Sun et al., 2003; Zhang et al., 2015; Zheng et al., 2020).
Bacterial phenotypic resistance to antibiotics generally increases in response to QACs, as measured by minimal inhibitory concentration (MIC) (Langsrud et al., 2004; McBain et al., 2004; Mechin et al., 1999). De novo mutation is an important way for bacteria to acquire antibiotic resistance under changing environmental conditions (Andersson and Hughes, 2014; Gullberg et al., 2011; Long et al., 2016). However, previous studies mostly focused on phenotypic resistance indicated by changes in MIC upon exposure of QACs at constant, subinhibitory concentrations. In a previous study, MICs to antibiotics increased by 3.5- to 7.5-fold after QACs exposure (Bore et al., 2007; Capita et al., 2019; Forbes et al., 2019; Maertens et al., 2020; Soumet et al., 2012). Only a few studies have attempted to attribute the phenotypic resistance to specific gene mutations. For example, knocking out the putative efflux pump gene sdeAB in QACs-resistant strains resulted in restored susceptibility to most QACs and antibiotics (Maseda et al., 2009). Examining de novo mutations in a high-throughput manner would allow better elucidation of QACs-induced resistance mechanism.
This study aimed to explore the relationship between increased resistance and de novo mutations caused by QACs, and investigate whether different mutations would be developed under constant subinhibitory concentration versus increasing inhibitory concentration of QACs. To achieve this objective, we characterized the phenotypic and genotypic resistance of E. coli in response to two most frequently used QACs, benzalkonium chloride (BAC, mono-chained) and didecyldimethylammonium chloride (DDAC, twin-chained). It was hypothesized that resistance induced by QACs were different according to the alkyl chain length of QACs that interact with cell membrane. Two sets of exposure experiments were conducted: continuous exposure to subinhibitory concentrations of QACs, and exposure to increasing inhibitory concentrations of QACs. The MICs of antibiotics were detected through the 60-day subculturing process. Mutations were identified by whole-genome sequencing to infer resistance mechanism. The stability of antibiotic resistance upon alleviating QACs stresses were evaluated. Outcomes from this study are expected to greatly improve current understandings of QACs-induced antibiotic resistance as a result of intensive disinfection, especially during the COVID-19 pandemic.
2. Materials and methods
2.1. QACs, antibiotics, and test strain
Two QACs, benzalkonium chloride (BAC, C6H5CH2N(CH3)2RCl (R = C8H17 to C18H37)) and didecyldimethylammonium chloride (DDAC, C22H48ClN) were purchased from Sigma-Aldrich (USA). Ampicillin (Amp), chloramphenicol (Chl), norfloxacin (Nor) were obtained from Aladin (China), and tetracycline (Tet) and rifampin (Rif) were from Macklin (China). The five antibiotics with different antibacterial mechanisms have been widely applied worldwide, and were representational in previous studies on antibiotic resistance (Table S1). The solvent used for dissolving BAC, DDAC, Amp, Nor, Tet, Rif was MillQ water, while the solvent for Chl was 95% ethanol.
The model bacterium used in this study was Escherichia coli K-12 MG1655 (E. coli). Luria-Bertani (LB) medium was used for culture growth. E. coli stock from −80 °C freezer was inoculated on LB agar plate using the streak plate method and incubated at 37 °C for 24 h. Then a single colony was isolated from the plate and subsequently grown in liquid LB for 12 h at 37 °C, and 150 rpm to reach an OD600 of 1.0. The cell suspension was used for subsequent experiments. All the experiments were performed with biological triplicates.
2.2. Determination of minimum inhibitory concentrations (MICs)
To identify the initial exposure concentrations of QACs, the MICs of DDAC and BAC were tested. Briefly, an overnight bacteria culture was diluted with sterile 0.9% NaCl solution to an OD600 of 0.1 using a spectrophotometer colorimetric (Shimadzu, Japan). Next, 96-well plates were prepared and 10 μL bacteria suspension was added to each well containing 90 μL of serially, 2-fold diluted BAC or DDAC in Mueller-Hinton Broth (MHB) medium. For the growth control group, 10 μL bacteria suspension was added to 90 μL MHB medium without QACs. For the negative control group, 10 μL sterile 0.9% NaCl solution was used instead of bacteria suspension. For the blank control group, only 100 μL of MHB medium was added. The plates were incubated at 37 °C for 24 h to measure the OD600 of each well using a microplate reader (Tecan, Austria). The MICs were determined as the concentration that inhibited growth by 90% (MIC90) based on OD600. For simplicity, we used MIC instead of MIC90 in the rest. The MIC value of the ancestor strain was defined as the initial MIC (MIC0). Detailed detection methods of reactive oxygen species (ROS) production, cell membrane permeability and transmission electron microscope (TEM) after exposure to QACs below or above MIC0 are described in Test S1-S2.
2.3. QACs exposure and bacteria subculturing
To determine any possible adaptation to QACs, the strains were exposed to increasing subinhibitory concentrations of BAC or DDAC, respectively. The initial exposure concentrations of QACs were based on 0, 0.25, 0.5, 1 × MIC0. The 48-well plates were used for bacterial culture. Specifically, 10 μL overnight culture of wild-type (WT) E. coli was firstly added to each well containing 490 μL of serially, 2-fold diluted QACs in LB medium. After growing at 37 °C and 150 rpm for 24 h, 10 μL of the suspension were taken from each well to the next well of a new plate containing the same concentration of QACs. When growth was observed at the highest inhibitory concentration the suspension was additionally transferred into a well with twice the previous concentration. The cultures were subcultured for 60 days and they eventually adapted to 2 × MIC0 of BAC or DDAC, respectively. During the 60-day exposure, the MIC fold changes of five antibiotics for E. coli that adapted to the highest concentrations of QACs at that time were measured every 5 days.
2.4. Isolation of resistant mutants and hereditary stability test
For the resistance mutant isolation, the last cell cultures under 0, 0.5, 2 × MIC0 of BAC or DDAC exposure were diluted 1:106 and spread on LB agar plates containing individual antibiotics at 1 × MIC0 (i.e., 16 mg/L Chl, 10 mg/L Tet, 8 mg/L Amp, 8 mg/L Rif, 0.5 mg/L Nor). The plates were incubated at 37 °C for 24 h. Colonies grown on the selective plates were considered as potential antibiotic resistance mutants. Three mutants were randomly picked up from each selective plate. The MICs of five antibiotics for these mutants were determined. Considering the same mutations of mutants selected from Amp and Nor plates, as well as the Chl and Rif plates, mutants selected from Chl, Tet and Amp plates were presented in the following sections.
According to MICs values, mutants with the highest resistance under 0.5, 2 × MIC0 of BAC or DDAC exposure were selected from Chl, Tet and Amp plates (namely 0.5BAC-Chl-R, 0.5BAC-Tet-R, 0.5BAC-Amp-R, 2BAC-Chl-R, 2BAC-Tet-R, 2BAC-Amp-R, 0.5DDAC-Chl-R, 0.5DDAC-Tet-R, 0.5DDAC-Amp-R, 2DDAC-Chl-R, 2DDAC-Tet-R, 2DDAC-Amp-R). Then they were diluted 1:1000 and subcultured in LB media without QACs at 37 °C and 150 rpm, the media were changed every 24 h. After 5 days, the MICs of five antibiotics for these resubcultured mutants were tested again to see whether the resistance were still well kept.
2.5. DNA extraction, whole-genome sequencing (WGS), and variant detection
Mutants with the highest resistance and untreated WT E. coli were cultured in 5 mL liquid LB at 37 °C and 150 rpm. Genomic DNA was extracted using TIANamp Bacteria DNA Kit (Tiangen, China). The harvested DNA was detected by the agarose gel electrophoresis and quantified by NanoDrop™ One (Thermo Scientific, USA).
Sequencing libraries were generated using NEBNext® Ultra™ DNA Library Prep Kit for Illumina (NEB, USA) following manufacturer's recommendations. The whole genome of WT E. coli was sequenced using Illumina HiSeq PE 150 platform at the Beijing Allwegene Technology Co., Ltd. All good quality paired reads were assembled using the SPAdes (v3.13.0) (Bankevich et al., 2012) into a number of scaffolds. Finally, scaffolds with larger than 500 bp were selected for subsequent analysis. The trimmed reads of WT E. coli were aligned against the E. coli K-12 MG1655 genome from Genbank (NC_000913.3) to assemble the WT E. coli genome.
All isolated mutants DNA was sequenced on an Illumina HiSeq 4000 instrument using 2 × 150 bp paired-end reads. After sequencing, efficient sequencing was compared with WT E. coli genome using BWA software and then marked the repeated reads using SAMtools (Li et al., 2009) software. The SAMtools was also used to detect single nucleotide polymorphism (SNP) and filter SNPs with PE reads less than 4. Annotation of SNP, Insertion/Deletion (InDel) used ANNOVAR (Wang et al., 2010) software. In addition, the statistical analysis was calculated and plotted using R and Perl.
2.6. Quantitative reverse transcription PCR (RT-qPCR)
Based on the SNP/InDel detection, the Chl-R, Tet-R and Amp-R mutants as well as WT E. coli were tested relatively genes expression by RT-qPCR. The strains were cultured in liquid LB with 0.8MIC0 of respective antibiotics (Chl for the Chl-R mutant, Tet for the Tet-R mutant, Amp for the Amp-R mutant) at 37 °C and 150 rpm for 6 h. Bacteria in logarithmic growth were harvested by centrifugation. The total RNA was extracted using a RNAprep Pure Cell/Bacteria Kit (Tiangen, China). RNA samples were converted to cDNA with a FastKing RT Kit (Tiangen, China). RT-qPCR was performed using SuperReal PreMix (SYBR Green) (Tiangen, China) with transcribed cDNA templates on StepOnePlus™ Real-Time PCR System (Applied Biosystems, USA) following the instruction. The primers were listed in the Table S2. The relative expression was calculated by the 2-△△Ct method and the results were showed as log2 fold change (LFC) (Livak and Schmittgen, 2001). All experiments in this study were carried out with independent biological replicates and repeated at least 3 times.
3. Results and discussion
3.1. Different levels of QACs exposure led to variations in multidrug resistance and stability
The MIC0 values of BAC and DDAC were 20 mg/L and 4.5 mg/L, respectively. ROS production and cell morphology changes in response to QACs at above-MIC0 levels were significant, whereas those under subinhibitory concentrations (0.25× and 0.5 × MIC0) were insignificant (Fig. S1a and S1c). As QACs are positively charged and act on cell membrane, cell membrane damage was observed at exposure concentration > 0.5 × MIC0 (Fig. S1b). Notably, all exposure concentrations were much lower than the critical micelle concentrations (BAC: 1.41 g/L, DDAC: 0.67 g/L), and thus QACs acted as single molecules or aggregation of several molecules in the solution (Test S3 and Fig. S2).
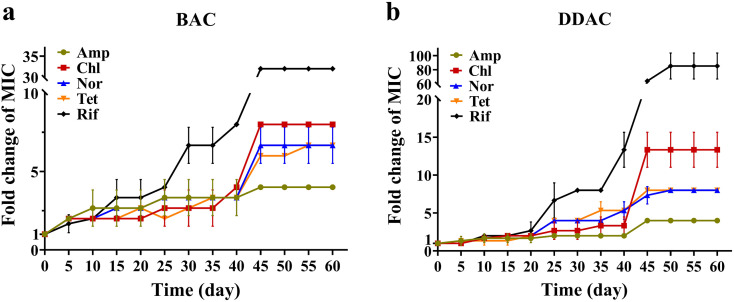
During the subculturing process, the rate of MIC increase was not constant (Fig. 1) . BAC exposure led to increased MIC on 3d (40 mg/L, 2 × MIC0) and 43d (80 mg/L, 4 × MIC0), and a similar pattern was observed during DDAC exposure on 21d (9 mg/L, 2 × MIC0) and 43d (18 mg/L, 4 × MIC0). The ultimate MIC values of BAC and DDAC were 80 mg/L and 18 mg/L (approximately 4 × MIC0), respectively. The initial MIC values of Amp, Chl, Nor, Tet and Rif against E. coli were 8, 16, 0.25, 10 and 8 mg/L, respectively. The MIC values of antibiotics increased rapidly from before to after these adaptations occurred (0–5d and 40–45d for BAC, 20–25d and 40–45d for DDAC). In the last 15 days (40–60d), the MIC values remained unchanged (Table S3). The final MIC values of Amp, Chl, Nor, Tet and Rif were 32, 128, 1.7 ± 0.3, 66.7 ± 11.5, 256 mg/L after BAC exposure, and 32, 213.3 ± 37.0, 2, 80, 682.7 ± 147.8 after DDAC exposure. The adaptation to BAC occurred faster than that of DDAC, and as expected, MIC values of the five tested antibiotics also increased earlier under BAC exposure than that of DDAC (0–20d). Compared to mono-chained BAC, twin-chained DDAC replaces the benzyl group of BAC with an additional long carbon side chain, exhibiting better antimicrobial activity, and thus the strains under BAC exposure adapt faster to QACs (Wang et al., 2018). However, in terms of resistance to antibiotics, the MIC values of Chl, Tet, and Rif under DDAC exposure increased to higher degrees than that of BAC exposure. Among the five antibiotics, Rif had the greatest variations in MIC values (increased to 32.00 and 85.33 ± 18.48 times of MIC0 under BAC and DDAC exposure, respectively). This indicated potentially stable acquisition of genetic resistance to Rif in addition to an expression of intrinsic resistance, which generally leads to a small increase in resistance.
Fig. 1.
Fold changes in the MIC values of E. coli subcultures against five antibiotics under BAC (a) and DDAC (b) exposure. Amp: ampicillin, Chl: chloramphenicol, Nor: norfloxacin, Tet: tetracycline, Rif: rifampin. The subcultures with the highest resistance to BAC and DDAC were tested for antibiotic resistance at each sampling point with biological triplicates.
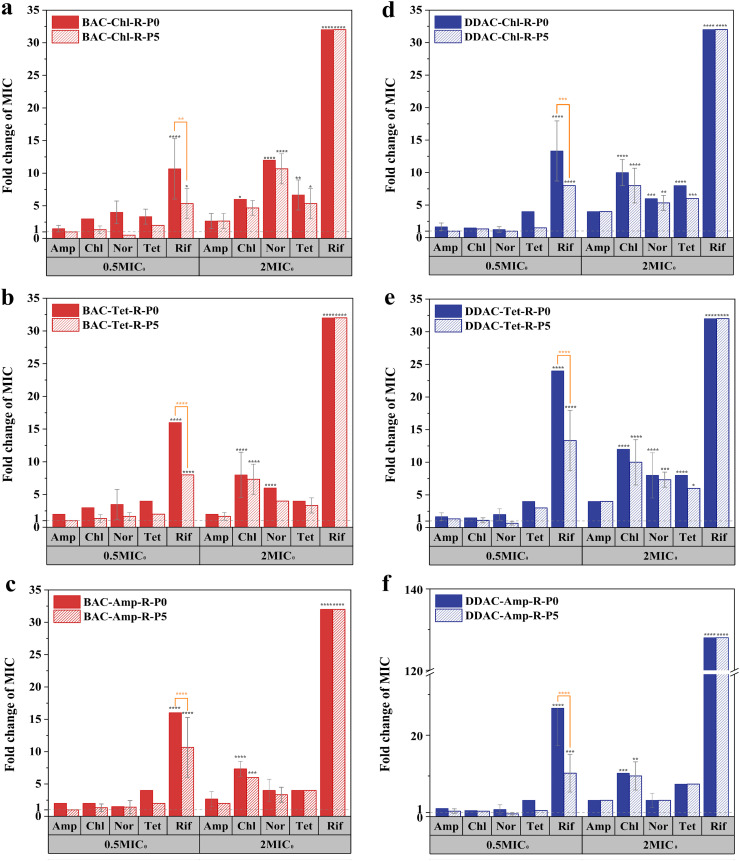
To investigate whether E. coli would lose the antibiotic resistance developed under different levels of QACs exposure, the mutants were cultivated for another 5 days without QACs (endpoint subculture: P5, Fig. 2 ). The 0.5 × MIC0-mutants exhibited high resistance to Rif (MIC values were 10.67 ± 4.62 to 26.67 ± 9.24 times of the WT strains, p < 0.0001), which decreased significantly in the P5 subcultures (MIC values were 5.33 ± 2.31 to 13.33 ± 4.62 times of the WT strains, p < 0.05), indicating a rapid loss of resistance. On the other hand, the high resistance of 2 × MIC0-mutants to multiple antibiotics remained in their P5 subcultures (Table S4). The resistance of 2 × MIC0-mutants was inheritable, and the mutagenic effects occurred during adaptation rather than exposure to QACs at subinhibitory concentrations. As bacteria proliferate, antibiotic resistance continuously increased, posing higher risks of gene transfer in the environment.
Fig. 2.
Antibiotic MICs of putative mutants BAC-Chl-R (a), BAC-Tet-R (b), BAC-Amp-R (c), DDAC-Chl-R (d), DDAC-Tet-R (e), DDAC-Amp-R (f) at the end of subculturing process (P0) and 5 days after cultivation in the absence of BAC or DDAC (P5) with biological triplicates. The mutants were isolated from antibiotic plates inoculated with subcultures exposed to BAC or DDAC at 0.5MIC and 2MIC for 60 days. Values on the y-axis represent fold changes in MIC compared to those of WT strains. Statistically significant differences were denoted by asterisks: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.2. Genetic mutations under QACs exposure
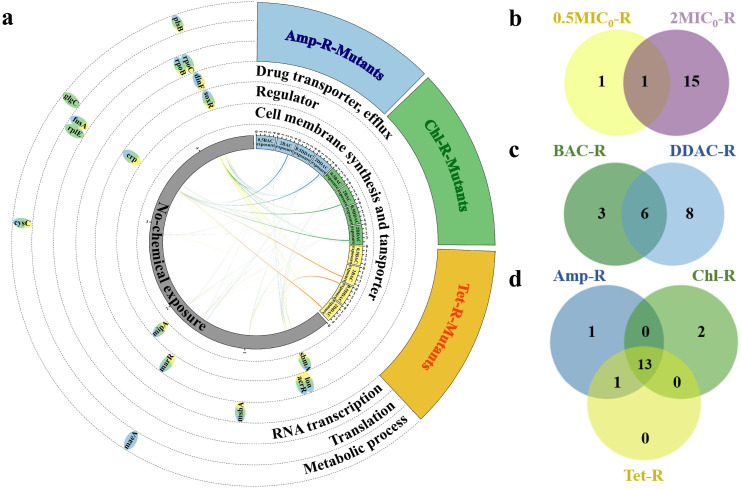
To understand the molecular mechanism of QACs-induced antibiotic resistance, the 0.5 × MIC0-mutants and 2 × MIC0-mutants with the highest MIC values against five antibiotics were selected for WGS. A total of 19 SNPs and 1 Indel (acrR indel mutation with 1-bp insertion resulted in a frameshift) in 17 genes were detected (Table S5). Except for the synonymous mutation of fusA, the remaining SNPs were nonsynonymous, which can be grouped into six categories according to their functions: (i) RNA transcription (rpoB, rpoC), (ii) regulator (acrR, marR, soxR, crp, lon), (iii) drug transporter, efflux pumps (msbA, dinF), (iv) cell membrane synthesis and transporter (mipA, sbmA), (v) translation (fusA, rplE) and (vi) metabolic processes (plsB, glgC, maeA, cysC) (Fig. 3a).
Fig. 3.
BAC and DDAC induced genetic mutations. (a) Genetic mutations identified in the sequenced resistance mutants. Mutated genes were grouped based on function and marked on the circular genome of E. coli. Yellow, blue, and green colors represent mutants isolated from antibiotic plates containing Tet, Amp, Chl, respectively; (b) Venn diagram showing the number of shared (brown) and unique mutated genes in 0.5MIC0-R (yellow) and 2MIC0-R (violet) mutants; (c) Venn diagram showing the number of gene mutations shared (cyan) and unique mutated genes in BAC-R (green) and DDAC-R (blue) mutants; (d) Venn diagram showing the number of gene mutations shared (light green) and unique mutated genes in Amp-R (blue), Chl-R (green) and Tet-R (yellow). Detailed information of the mutated genes can be found in Table S4.
2 × MIC0-mutants harbored more mutated genes than the 0.5 × MIC0-mutants (16 v.s. 2, Fig. 3b and Table S5). E. coli inclined to acquire resistance through gene mutations during the adaptation to QACs. Mutation is one of the basic processes by which genetic variation emerges in bacterial populations (Didelot and Maiden, 2010). 2 × MIC0-mutants could pass on their resistance to offsprings without continued QACs exposure (Fig. 2). In contrast, the resistance of 0.5 × MIC0-mutants may originate from upregulated expression of intrinsic resistance under QACs, and the cells returned to the sensitive state upon withdrawal of QACs stresses.
BAC and DDAC exposure commonly induced 6 mutations in rpoB and ropC encoding RNA polymerase (RNAP), and acrR, marR, soxR, crp encoding the regulators of AcrAB-TolC and MdtEF efflux pumps (Fig. 3c). All of these genes have documented associations with antibiotic resistance (discussed in Section 3.3). In addition, the mutated genes induced by DDAC included msbA, dinF, fusA, rplE, plsB, glgC, maeA and cysC, which were mainly related to drug transport, bacterial protein synthesis and metabolism. BAC induced mutations in mipA, sbmA and lon, which were mainly related to cell membrane structure, transporter, and efflux related proteins. The different antibiotics used for screening mutants had limited effects on gene mutations (Fig. 3d), indicating that mutations did occur during the 960-generation, 60-day subculturing process.
3.3. Association of mutated genes with antibiotic resistance
Among the mutated genes identified, rpoB encodes the beta subunit of RNAP, which is the target of rifampicin (Jindani et al., 2003). It was reported that rpoB could evolve quickly upon rifampicin exposure (Goldstein, 2014). Mutations in specific positions of the coding sequences are known to affect the binding of drugs to RNAP, leading to drug resistance (Haunreiter et al., 2019). Mutations in rpoB burdened a fitness cost in the absence of antibiotics, while compensatory mutations in rpoC could restore the fitness of rifampin-resistant bacteria (Brandis et al., 2012; Comas et al., 2012; de Vos et al., 2013; Tawgozy and Qarasnji, 2020). The specific SNPs detected in rpoB (Asp342 → Gly) and rpoC (Ala787 → Val) have not been described so far. However, the same site was mutated (Ala787 → Thr) in a plasmid-borne rpoC constructed for E. coli (Ito and Nakamura, 1996). These mutations occurred in 2BAC-R and 2DDAC-R strains (Table S5), and they were deemed to play important roles in acquiring heritable rifampicin resistance (Fig. 4c).
Fig. 4.
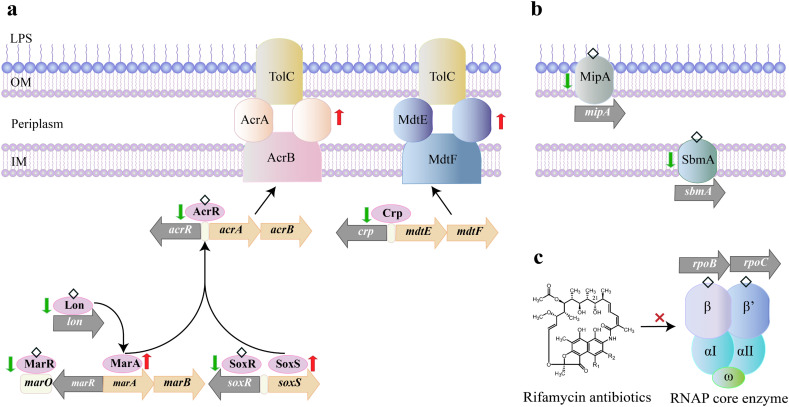
Proposed antibiotic resistance mechanism of QACs-induced E. coli mutants: (a) stimulated efflux expression; (b) impeded transmembrane transport; (c) modified antibacterial targets. Mutated genes were marked in gray. Proteins (in ellipses) with changes in amino acid sequences were denoted by diamonds. Green/red arrows represent down/up-regulations in gene expressions. LPS: lipopolysaccharide, OM: outer membrane, IM: inner membrane.
Another major class of mutated genes were regulators of multidrug efflux pumps, e.g., AcrAB-TolC (acrR, soxR, marR) and MdtEF (crp). AcrAB-TolC, a tripartite system consisting of a periplasmic protein (AcrA), a transporter protein (AcrB) and an accessory protein channel (TolC), is the main multidrug efflux machinery in E. coli, endowing bacteria with resistance to broad-spectrum antibiotics (Du et al., 2015; Okusu et al., 1996; Ramos et al., 2014). The expression of acrAB was regulated by local repressor genes, including the adjacent acrR gene, as well as by marR, and soxR genes. Mutations in these genes might result in acrAB overexpression and multidrug resistance (Bialek-Davenet et al., 2011). In this study, two mutations in acrR occurred in 0.5DDAC-Tet-R (Trp4 → Arg) and BAC-induced mutants (except 0.5BAC-R and 2BAC-Amp-R, Table S5). The specific SNPs in soxR (Leu95 → Ser) and marR (Val45 → Glu) were detected in 2BAC-R and 2DDAC-R mutants (Table S5). Furthermore, the mutation in lon (Lys35 → Glu) detected in 2BAC-R mutants was also considered to influence the expression of acrAB, because MarA, a positive regulator governing acrAB expression, was identified as a downstream target of Lon protease (Bhaskarla et al., 2016). Similarly, the mutations in crp, a repressor of MdtEF, might increase the expression of mdtEF genes, leading to antibiotics extrusion (Hirakawa et al., 2006). The variations in crp occurred in 2DDAC-R (Asp54 → Gly), 2BAC-R (Gln171 → Lys), 2BAC-R and 2DDAC-R (Phe63 → Ser) (Table S5). These mutations have not been reported before.
Mutations also occurred in drug transporter genes. The 2DDAC-R mutants had a mutation in the msbA (Ala247 → Val) gene (Table S5). It encodes the inner membrane ATP-binding cassette (ABC) transporter MsbA, which can extrude antibiotics and toxic ions from the cell (Alexander et al., 2018; Reuter et al., 2003; Woebking et al., 2008). It has been reported that mutations in the msbA gene of Pseudomonas aeruginosa were related to antibiotic resistance (Diez-Aguilar et al., 2021). Therefore, we suspected the increased antibiotic resistance could be partially attributed to the msbA mutation in E. coli. Another mutated gene related to drug transport was dinF, encoding the DNA damage-inducible protein F, a member of the multi-antimicrobial extrusion superfamily associated with SOS response (Brown et al., 2007). The mutations of the dinF gene exhibited increasing susceptibility to quinolones antibiotics and low ability to grow in the presence of various toxic compounds (e.g., antibiotics, phytoalexins, and detergents) (Brown et al., 2007; Tocci et al., 2013). Conversely, in our study, the mutants of 2DDAC-Amp-R and 2DDAC-Tet-R with mutation in dinF (Leu339 → Ile) had increased resistance to ciprofloxacin and other antibiotics (Table S4). A recent study showed that in the absence of dinF, E. coli were tolerant to periplanetasin-4 (a novel antimicrobial peptide), which damaged DNA and initiated SOS response by accumulating nitric oxide (a free radical with high toxicity) (Lee et al., 2021). In this context, the dinF gene mutation may have similar consequences by influencing the intracellular damage pathway which deserves experimental validation.
The mutations in mipA (Gly31 → Asp) and sbmA (Arg37 → Pro) in 2BAC-R mutants were associated with cell membrane structure and transporters (Table S5). mipA encodes an outer membrane protein called MltA-interacting protein (MipA), a novel antibiotic resistance-related protein. The deletion of mipA in E. coli resulted in the increase in MICs of kanamycin, chloramphenicol and aureomycin (Dan-feng et al., 2015). The sbmA gene encodes an inner membrane protein (SbmA) that contains eight transmembrane regions, which can transport peptide antibiotics into cytoplasm, and thus its mutation may increase the resistance to these antibiotics (Corbalan et al., 2013; Cristobal et al., 2008; Mattiuzzo et al., 2007).
fusA and rplE genes are associated with translation. Mutations in fusA, which encodes the drug target of antibiotic fusidic acid (elongation factor G), could be related to antibiotic resistance (O'Neill et al., 2007). Nevertheless, the fusA mutation in 2DDAC-R mutant was synonymous (Table S5), and thereby its correlation with increased antibiotic resistance was weak. rplE encoding the 50S ribosomal subunit protein L5 is important to cell viability, whereas its relationship with antibiotic resistance has not been reported.
QACs induced various non-canonical mutations of metabolic genes. plsB gene is involved in glycerol-3-phosphate metabolism, which plays an important role in phospholipid synthesis and persister formation (a specialized cell state that exhibit multidrug tolerance, including the ability to survive by antibiotics killing) (Lewis, 2008; Spoering et al., 2006). plsB gene mutation could diminish the rate of phospholipid synthesis, resulting in dormancy and increase in the level of persisters (Spoering et al., 2006). Based on this, we suspected the specific SNP in plsB (Gly130 → Ser) occurring in 2DDAC-R mutants may contribute to the increase of antibiotic resistance (Table S5). The exact mechanism deserves further investigation. Mutations in glgC, maeA and cysC, which encode ADP-glucose pyrophosphorylase, malate dehydrogenase and adenylyl-sulfate kinase, respectively, may significantly affect metabolism. For example, the mutations in glgC could empower evolved bacteria with abilities to inhibit or kill their ancestors (Lemonnier et al., 2008). Similarly, a clear link between mutations of these genes and antibiotic resistance is lacking.
3.4. Expression of efflux related genes
Based on the association of mutated genes with antibiotic resistance, we hypothesized that certain genetic mutations could influence gene expressions contributing to antibiotic resistance, especially those related to efflux pumps and cell membrane structure (Fig. 4a-b). To verify this hypothesis, we measured the expression levels of mutated genes involving (i) regulator, (ii) drug transporter and efflux, and (iii) cell membrane structure and transporter.
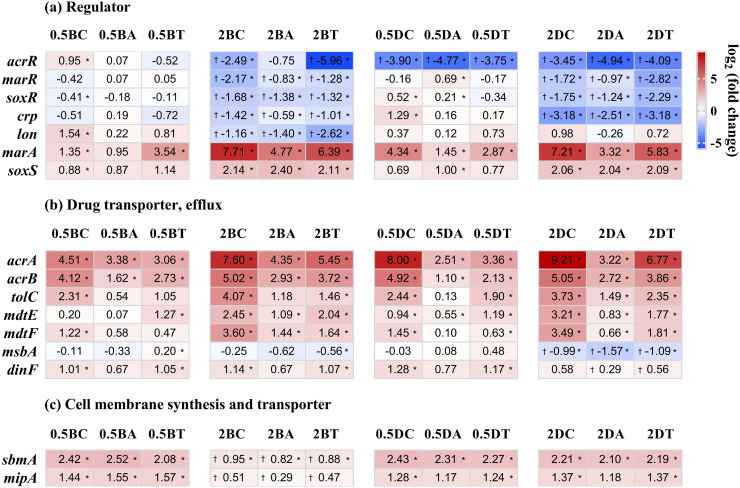
Antibiotics extrusion is an intrinsic resistance mechanism of E. coli, i.e., strains without mutations of efflux pump related genes could also have up-regulated expressions of these genes (Fig. 5b). On the other hand, mutations of negative regulators can enhance the expression of this intrinsic resistance. This occurred during the subculturing or adaptation of E. coli to QACs. Specifically, mutated regulator genes (acrR, marR, soxR, crp, lon) showed decreases in expression, but the expression of positive regulatory genes (marA, soxS) acting on the downstream of these regulators increased (Fig. 5a). As a result, the expression of genes coding for multidrug efflux pumps (acrA, acrB, tolC, mdtE, mdtF) increased (Fig. 5b). For example, gene acrR, a negative regulator of AcrAB-TolC efflux, showed a decreased LFC of −3.90, −4.77, −3.75, −2.49, −0.75, −5.96, −3.45, −4.94, −4.09 in the 0.5DDAC-R, 2BAC-R, 2DDAC-R mutants, respectively, while its expression in the 0.5BAC-R mutant without acrR mutation remained unchanged (Fig. 5a). The expressions of other negative regulatory genes were downregulated (marR, soxR in 2BAC-R and 2DDAC-R; lon in 2BAC-R) (Fig. 4a). In contrast, the positive regulatory genes (marA, soxS) were up-regulated. Genes coding for AcrAB-TolC efflux pumps (acrA, acrB, tolC) were upregulated. Similarly, the genes related to MdtEF efflux pumps (mdtE, mdtF) had higher expression in 2BAC-R and 2DDAC-R with mutations in the negative regulatory gene crp, which itself showed decreased expression (Fig. 5a-b).
Fig. 5.
The expressions of selected mutated genes related to regulator (a), drug transporter and efflux (b), and cell membrane synthesis and transporter (c) in mutants with biological triplicates. Red/blue indicate up/down-regulated genes. Log2 fold change |LFC| ≥2 and *p < 0.05 are the criteria for differentially expressed genes. The dagger (†) represents strains harboring specific mutation. The abbreviations of mutants provided the information regarding QACs, exposure concentration, and antibiotics. Specifically, 0.5 BC: 0.5BAC-Chl-R, 0.5BA: 0.5BAC-Amp-R, 0.5BT: 0.5BAC-Tet-R, 2 BC: 2BAC-Chl-R, 2BA: 2BAC-Amp-R, 2BT: 2BAC-Tet-R, 0.5 DC: 0.5DDAC-Chl-R, 0.5DA: 0.5DDAC-Amp-R, 0.5DT: 0.5DDAC-Tet-R, 2 DC: 2DDAC-Chl-R, 2DA: 2DDAC-Amp-R, 2DT: 2DDAC-Tet-R.
The LFC reduced to −0.99, −1.57, −1.09 for msbA and 0.58, 0.26, 0.56 for dinF (Fig. 5b). This appeared to contradict our findings of increasing antibiotic resistance in 2DDAC-R mutants, as MsbA and DinF were responsible for extruding antibiotics (Alexander et al., 2018; Brown et al., 2007; Reuter et al., 2003; Tocci et al., 2013; Woebking et al., 2008). Additionally, the |LFC| values of these genes were below 2. Therefore, we suspected that AcrAB-TolC and MdtEF efflux pumps, rather than the two drug transporters, played a major role in extruding antibiotics. LFC of 2BAC-R with sbmA and mipA mutations decreased to 0.95, 0.82, 0.88 (sbmA) and 0.51, 0.29, 0.47 (mipA) although the expression still increased compared to the WT strain (Fig. 5c). The above results indicated that mutations in sbmA and mipA led to decreases in their expressions, which may in turn reduce the transport of antibiotics via these two membrane transporters.
4. Conclusions
Compared with mono-chained BAC, twin-chained DDAC led to slower development but higher level of antibiotic resistance in E. coli. Mutated genes conferring QAC-induced antibiotic resistance were associated with negative regulators (acrR, marR, soxR, crp), cell membrane synthesis and transporters (mipA, sbmA), and RNAP (rpoB, rpoC). At sub-MIC concentrations, QACs-induced antibiotic resistance arose through upregulated gene expressions was largely reversible when QACs stresses were removed. Exposure at 2 × MIC resulted in mutations that facilitated the development of stronger and irreversible resistance. DDAC with long alkyl chain had higher hydrophobicity, stronger antibacterial activity, more gene mutations and higher resistance levels, which may have a greater impact on the enhancement of environmental resistance. The evolution and potential propagation of antibiotic resistance in response to increasing environmental concentrations of QACs during the pandemic, in particular DDAC, deserves critical attention.
CRediT authorship contribution statement
Yin Jia: Data curation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Huijie Lu: Investigation, Visualization, Writing – original draft, Writing – review & editing. Lizhong Zhu: Conceptualization, Investigation, Funding acquisition, Supervision, Project administration, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (22193061 and 21836003) and National Key Research and Development Program of China (2020YFC1806903).
Editor: Fang Wang
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2022.155090.
Appendix A. Supplementary data
Supplementary material
References
- Alexander M.K., Miu A., Oh A., Reichelt M., Ho H.D., Chalouni C., Labadie S., Wang L., Liang J., Nickerson N.N., Hu H.Y., Yu L., Du M.F., Yang D.H., Park S., Kim J., Xu M., Sellers B.D., Purkey H.E., Skelton N.J., Koehler M.F.T., Payandeh T., Verma V., Xu Y.M., Koth C.M., Nishiyama M. Disrupting gram-negative bacterial outer membrane biosynthesis through inhibition of the lipopolysaccharide transporter MsbA. Antimicrob. Agents Chemother. 2018;62:15. doi: 10.1128/aac.01142-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alygizakis N., Galani A., Rousis N.I., Aalizadeh R., Dimopoulos M.A., Thomaidis N.S. Change in the chemical content of untreated wastewater of Athens, Greece under COVID-19 pandemic. Sci. Total Environ. 2021;799:10. doi: 10.1016/j.scitotenv.2021.149230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson D.I., Hughes D. Microbiological effects of sublethal levels of antibiotics. Nat. Rev. Microbiol. 2014;12:465–478. doi: 10.1038/nrmicro3270. [DOI] [PubMed] [Google Scholar]
- Baker-Austin C., Wright M.S., Stepanauskas R., McArthur J.V. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006;14:176–182. doi: 10.1016/j.tim.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskarla C., Das M., Verma T., Kumar A., Mahadevan S., Nandi D. Roles of lon protease and its substrate MarA during sodium salicylate-mediated growth reduction and antibiotic resistance in Escherichia coli. Microbiol.Sgm. 2016;162:764–776. doi: 10.1099/mic.0.000271. [DOI] [PubMed] [Google Scholar]
- Bialek-Davenet S., Marcon E., Leflon-Guibout V., Lavigne J.-P., Bert F., Moreau R., Nicolas-Chanoine M.-H. In vitro selection of ramR and soxR mutants overexpressing efflux systems by fluoroquinolones as well as cefoxitin in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2011;55:2795–2802. doi: 10.1128/aac.00156-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bore E., Hebraud M., Chafsey I., Chambon C., Skjaeret C., Moen B., Moretro T., Langsrud O., Rudi K., Langsrud S. Adapted tolerance to benzalkonium chloride in Escherichia coli K-12 studied by transcriptome and proteome analyses. Microbiolol.Sgm. 2007;153:935–946. doi: 10.1099/mic.0.29288-0. [DOI] [PubMed] [Google Scholar]
- Brandis G., Wrande M., Liljas L., Hughes D. Fitness-compensatory mutations in rifampicin-resistant RNA polymerase. Mol. Microbiol. 2012;85:142–151. doi: 10.1111/j.1365-2958.2012.08099.x. [DOI] [PubMed] [Google Scholar]
- Brown D.G., Swanson J.K., Allen C. Two host-induced ralstonia solanacearum genes, acrA and dinF, encode multidrug efflux pumps and contribute to bacterial wilt virulence. Appl. Environ. Microbiol. 2007;73:2777–2786. doi: 10.1128/aem.00984-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capita R., Vicente-Velasco M., Rodríguez-Melcón C., García-Fernández C., Carballo J., Alonso-Calleja C.J.S.R. Effect of low doses of biocides on the antimicrobial resistance and the biofilms of Cronobacter sakazakii and Yersinia enterocolitica. Sci. Rep. 2019;9:15905. doi: 10.1038/s41598-019-51907-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clara M., Scharf S., Scheffknecht C., Gans O. Occurrence of selected surfactants in untreated and treated sewage. Water Res. 2007;41:4339–4348. doi: 10.1016/j.watres.2007.06.027. [DOI] [PubMed] [Google Scholar]
- Comas I., Borrell S., Roetzer A., Rose G., Malla B., Kato-Maeda M., Galagan J., Niemann S., Gagneux S. Whole-genome sequencing of rifampicin-resistant mycobacterium tuberculosis strains identifies compensatory mutations in RNA polymerase genes. Nat. Genet. 2012;44:106–147. doi: 10.1038/ng.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbalan N., Runti G., Adler C., Covaceuszach S., Ford R.C., Lamba D., Beis K., Scocchi M., Vincent P.A. Functional and structural study of the dimeric inner membrane protein SbmA. J. Bacteriol. 2013;195:5352–5361. doi: 10.1128/jb.00824-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristobal R.E.d., Vincent P.A., Salomon R.A. A combination of sbmA and tolC mutations in Escherichia coli K-12 Tn10-carrying strains results in hypersusceptibility to tetracycline. J. Bacteriol. 2008;190:1491–1494. doi: 10.1128/jb.01844-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dan-feng Z., Hui L., Xiang-min L., Xuan-xian P. Outer membrane proteomics of kanamycin-resistant Escherichia coli identified MipA as a novel antibiotic resistance-related protein. FEMS Microbiol. Lett. 2015;362 doi: 10.1093/femsle/fnv074. [DOI] [PubMed] [Google Scholar]
- Didelot X., Maiden M.C.J. Impact of recombination on bacterial evolution. Trends Microbiol. 2010;18:315–322. doi: 10.1016/j.tim.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Aguilar M., Hernandez-Garcia M., Morosini M.I., Fluit A., Tunney M.M., Huertas N., Del Campo R., Obrecht D., Bernardini F., Ekkelenkamp M., Canton R. Murepavadin antimicrobial activity against and resistance development in cystic fibrosis Pseudomonas aeruginosa isolates. J. Antimicrob. Chemother. 2021;76:984–992. doi: 10.1093/jac/dkaa529. [DOI] [PubMed] [Google Scholar]
- Du D., van Veen H.W., Luisi B.F. Assembly and operation of bacterial tripartite multidrug efflux pumps. Trends Microbiol. 2015;23:311–319. doi: 10.1016/j.tim.2015.01.010. [DOI] [PubMed] [Google Scholar]
- Ferrer I., Furlong E.T. Identification of alkyl dimethylbenzylammonium surfactants in water samples by solid-phase extraction followed by ion trap LC/MS and LC/MS/MS. Environ. Sci. Technol. 2001;35:2583–2588. doi: 10.1021/es001742v. [DOI] [PubMed] [Google Scholar]
- Forbes S., Morgan N., Humphreys G.J., AmeZquita A., Mistry H., McBain A.J. Loss of function in Escherichia coli exposed to environmentally relevant concentrations of benzalkonium chloride. Appl. Environ. Microbiol. 2019;85:14. doi: 10.1128/aem.02417-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B.P. Resistance to rifampicin: a review. J. Antibiot. 2014;67:625–630. doi: 10.1038/ja.2014.107. [DOI] [PubMed] [Google Scholar]
- Gullberg E., Cao S., Berg O.G., Ilback C., Sandegren L., Hughes D., Andersson D.I. Selection of resistant bacteria at very low antibiotic concentrations. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haunreiter V.D., Boumasmoud M., Haffner N., Wipfli D., Leimer N., Rachmuhl C., Kuhnert D., Achermann Y., Zbinden R., Benussi S., Vulin C., Zinkernagel A.S. In-host evolution of Staphylococcus epidermidis in a pacemaker-associated endocarditis resulting in increased antibiotic tolerance. Nat. Commun. 2019;10:14. doi: 10.1038/s41467-019-09053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa H., Inazumi Y., Senda Y., Kobayashi A., Hirata T., Nishino K., Yamaguchi A. N-acetyl-d-glucosamine induces the expression of multidrug exporter genes, mdtEF, via catabolite activation in Escherichia coli. J. Bacteriol. 2006;188:5851–5858. doi: 10.1128/jb.00301-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hora P.I., Pati S.G., McNamara P.J., Arnold W.A. Increased use of quaternary ammonium compounds during the SARS-CoV-2 pandemic and beyond: consideration of environmental implications. Environ. Sci. Technol. Lett. 2020;7:622–631. doi: 10.1021/acs.estlett.0c00437. [DOI] [PubMed] [Google Scholar]
- Ito K., Nakamura Y. Localization of nusA -suppressing amino acid substitutions in the conserved regions of the β′ subunit of Escherichia coli RNA polymerase. Mol. Gen. Genet. 1996;251:699–706. doi: 10.1007/BF02174119. [DOI] [PubMed] [Google Scholar]
- Jennings M.C., Minbiole K.P.C., Wuest W.M. Quaternary ammonium compounds: an antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015;1(7):288–303. doi: 10.1021/acsinfecdis.5b00047. [DOI] [PubMed] [Google Scholar]
- Jindani A., Dore C.J., Mitchison D.A. Bactericidal and sterilizing activities of antituberculosis drugs during the first 14 days. Am. J. Respir. Crit. Care Med. 2003;167:1348–1354. doi: 10.1164/rccm.200210-1125OC. [DOI] [PubMed] [Google Scholar]
- Kreuzinger N., Fuerhacker M., Scharf S., Uhl M., Gans O., Grillitsch B. Methodological approach towards the environmental significance of uncharacterized substances — quaternary ammonium compounds as an example. Desalination. 2007;215:209–222. doi: 10.1016/j.desal.2006.10.036. [DOI] [Google Scholar]
- Langsrud S., Sundheim G., Holck A.L. Cross-resistance to antibiotics of Escherichia coli adapted to benzalkonium chloride or exposed to stress-inducers. J. Appl. Microbiol. 2004;96:201–208. doi: 10.1046/j.1365-2672.2003.02140.x. [DOI] [PubMed] [Google Scholar]
- Lee H., Hwang J.S., Lee D.G. dinF elicits nitric oxide signaling induced by Periplanetasin-4 from American cockroach in Escherichia coli. Curr. Microbiol. 2021;78:3550–3561. doi: 10.1007/s00284-021-02615-5. [DOI] [PubMed] [Google Scholar]
- Lemonnier M., Levin B.R., Romeo T., Garner K., Baquero M.-R., Mercante J., Lemichez E., Baquero F., Blazquez J. The evolution of contact-dependent inhibition in non-growing populations of Escherichia coli. Proc. R. Soc. BBiol. Sci. 2008;275:3–10. doi: 10.1098/rspb.2007.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. In: Bacterial Biofilms. Romeo T., editor. Springer-Verlag; Berlin, Berlin: 2008. Multidrug tolerance of biofilms and persister cells; pp. 107–131. [DOI] [PubMed] [Google Scholar]
- Li X., Brownawell B.J. Quaternary ammonium compounds in urban estuarine sediment environments - a class of contaminants in need of increased attention? Environ. Sci. Technol. 2010;44:7561–7568. doi: 10.1021/es1011669. [DOI] [PubMed] [Google Scholar]
- Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., Genome Project Data P. The sequence alignment/map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zeng S.Y., He M., Gu A.Z. Water disinfection byproducts induce antibiotic resistance-role of environmental pollutants in resistance phenomena. Environ. Sci. Technol. 2016;50:3193–3201. doi: 10.1021/acs.est.5b05113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Long H., Miller S.F., Strauss C., Zhao C., Cheng L., Ye Z., Griffin K., Te R., Lee H., Chen C.-C., Lynch M. Antibiotic treatment enhances the genome-wide mutation rate of target cells. Proc. Natl. Acad. Sci. U. S. A. 2016;113:2498–2505. doi: 10.1073/pnas.1601208113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maertens H., Demeyere K., De Reu K., Dewulf J., Vanhauteghem D., Van Coillie E., Meyer E. Effect of subinhibitory exposure to quaternary ammonium compounds on the ciprofloxacin susceptibility of Escherichia coli strains in animal husbandry. BMC Microbiol. 2020;20:11. doi: 10.1186/s12866-020-01818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney A.R., Safaee M.M., Wuest W.M., Furst A.L. The silent pandemic: Emergent antibiotic resistances following the global response to SARS-CoV-2. iScience. 2021;24:9. doi: 10.1016/j.isci.2021.102304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Carballo E., Gonzalez-Barreiro C., Sitka A., Kreuzinger N., Scharf S., Gans O. Determination of selected quaternary ammonium compounds by liquid chromatography with mass spectrometry. Part II. Application to sediment and sludge samples in Austria. Environ. Pollut. 2007;146:543–547. doi: 10.1016/j.envpol.2006.07.016. [DOI] [PubMed] [Google Scholar]
- Maseda H., Hashida Y., Konaka R., Shirai A., Kourai H. Mutational upregulation of a resistance-nodulation-cell division-type multidrug efflux pump, SdeAB, upon exposure to a biocide, cetylpyridinium chloride, and antibiotic resistance in Serratia marcescens. Antimicrob. Agents Chemother. 2009;53:5230–5235. doi: 10.1128/aac.00631-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzo M., Bandiera A., Gennaro R., Benincasa M., Pacor S., Antcheva N., Scocchi M. Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol. 2007;66:151–163. doi: 10.1111/j.1365-2958.2007.05903.x. [DOI] [PubMed] [Google Scholar]
- McBain A.J., Ledder R.G., Moore L.E., Catrenich C.E., Gilbert P. Effects of quarternary-amonium based formulations on bacterial community dynamics and antimicrobial susceptibility. Appl. Environ. Microbiol. 2004;70:3449–3456. doi: 10.1128/aem.70.6.3449-3456.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechin L., Dubois-Brissonnet F., Heyd B., Leveau J.Y. Adaptation of Pseudomonas aeruginosa ATCC 15442 to didecyldimethylammonium bromide induces changes in membrane fatty acid composition and in resistance of cells. J. Appl. Microbiol. 1999;86:859–866. doi: 10.1046/j.1365-2672.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- Okusu H., Ma D., Nikaido H. AcrAB efflux pump plays a major role in the antibiotic resistance phenotype of Escherichia coli multiple-antibiotic-resistance (Mar) mutants. J. Bacteriol. 1996;178:306–308. doi: 10.1128/jb.178.1.306-308.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill A.J., McLaws F., Kahlineter G., Henriksen A.S., Chopra I. Genetic basis of resistance to fusidic acid in staphylococci. Antimicrob. Agents Chemother. 2007;51:1737–1740. doi: 10.1128/AAC.01542-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos P.I., Picão R.C., Almeida L.G., Lima N.C., Girardello R., Vivan A.C., Xavier D.E., Barcellos F.G., Pelisson M., Vespero E.C., Médigue C., Vasconcelos A.T., Gales A.C., Nicolás M.F. Comparative analysis of the complete genome of KPC-2-producing Klebsiella pneumoniae Kp13 reveals remarkable genome plasticity and a wide repertoire of virulence and resistance mechanisms. BMC Genom. 2014;15:54. doi: 10.1186/1471-2164-15-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G., Janvilisri T., Venter H., Shahi S., Balakrishnan L., van Veen H.W. The ATP binding cassette multidrug transporter LmrA and lipid transporter MsbA have overlapping substrate specificities. J. Biol. Chem. 2003;278:35193–35198. doi: 10.1074/jbc.M306226200. [DOI] [PubMed] [Google Scholar]
- Soumet C., Fourreau E., Legrandois P., Maris P. Resistance to phenicol compounds following adaptation to quaternary ammonium compounds in Escherichia coli. Vet. Microbiol. 2012;158:147–152. doi: 10.1016/j.vetmic.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Spoering A.L., Vulic M., Lewis K. GlpD and PlsB participate in persister cell formation in eschetichia coli. J. Bacteriol. 2006;188:5136–5144. doi: 10.1128/jb.00369-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H.F., Takata A., Hata N., Kasahara I., Taguchi S. Transportation and fate of cationic surfactant in river water. J. Environ. Monit. 2003;5:891–895. doi: 10.1039/b308988f. [DOI] [PubMed] [Google Scholar]
- Tandukar M., Oh S., Tezel U., Konstantinidis K.T., Pavlostathis S.G. Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environ. Sci. Technol. 2013;47:9730–9738. doi: 10.1021/es401507k. [DOI] [PubMed] [Google Scholar]
- Tawgozy F.H.A., Qarasnji B.K. Molecular analysis of rifampicin resistance conferring mutations in mycobacterium tuberculosis. Baghdad Sci. J. 2020;17:444–451. doi: 10.21123/bsj.2020.17.2.0444. [DOI] [Google Scholar]
- Tocci N., Iannelli F., Bidossi A., Ciusa M.L., Decorosi F., Viti C., Pozzi G., Ricci S., Oggioni M.R. Functional analysis of pneumococcal drug efflux pumps associates the MATE DinF transporter with quinolone susceptibility. Antimicrob. Agents Chemother. 2013;57:248–253. doi: 10.1128/aac.01298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Environmental Protection Agency List N: Disinfectants for Use Against SARS-CoV-2. 2022. https://www.epa.gov/pesticide-registration/list-n-disinfectants-use-against-sars-cov-2
- de Vos M., Mueller B., Borrell S., Black P.A., van Helden P.D., Warren R.M., Gagneux S., Victor T.C. Putative compensatory mutations in the rpoC gene of rifampin-resistant mycobacterium tuberculosis are associated with ongoing transmission. Antimicrob. Agents Chemother. 2013;57:827–832. doi: 10.1128/aac.01541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Li M.Y., Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:7. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.P., Wang H.H., Ren B.A., Li X.D., Wang L., Zhou H., Weir M.D., Zhou X.D., Masri R.M., Oates T.W., Cheng L., Xu H.H.K. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018;8:11. doi: 10.1038/s41598-018-23831-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lu J., Engelstadter J., Zhang S., Ding P.B., Mao L.K., Yuan Z.G., Bond P.L., Guo J.H. Non-antibiotic pharmaceuticals enhance the transmission of exogenous antibiotic resistance genes through bacterial transformation. ISME J. 2020;14:2179–2196. doi: 10.1038/s41396-020-0679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woebking B., Velamakanni S., Federici L., Seeger M.A., Murakami S., van Veen H.W. Functional role of transmembrane helix 6 in drug binding and transport by the ABC transporter MsbA. Biochemistry. 2008;47:10904–10914. doi: 10.1021/bi800778d. [DOI] [PubMed] [Google Scholar]
- Zhang C., Cui F., Zeng G.M., Jiang M., Yang Z.Z., Yu Z.G., Zhu M.Y., Shen L.Q. Quaternary ammonium compounds (QACs): a review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015;518–519:352–362. doi: 10.1016/j.scitotenv.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Zheng G., Filippelli G.M., Salamova A. Increased indoor exposure to commonly used disinfectants during the COVID-19 pandemic. Environ. Sci. Technol. Lett. 2020;7:760–765. doi: 10.1021/acs.estlett.0c00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material