Abstract
Background and Purpose:
Cognitive impairment occurs in 20%–40% of stroke patients and is a predictor of long-term morbidity and mortality. In this study, we aim to determine the association between poststroke cognitive impairment and stroke recurrence risk, in patients with anterior versus posterior circulation intracranial stenosis.
Methods:
This is a post-hoc analysis of the Stenting and Aggressive Medical Therapy for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial. The primary predictor was poststroke cognitive function measured by Montreal Cognitive Assessment (MOCA) at 3–6 months and the primary outcome was recurrent ischemic stroke. We used univariate and multivariable cox-regression models to determine the associations between MOCA at 3–6 months and recurrent stroke.
Results:
Of the 451 patients enrolled in SAMMPRIS, 393 patients met the inclusion criteria. The mean age of the sample (in years) was 59.5 ± 11.3, 62.6% (246 of 393) were men. Fifty patients (12.7%) had recurrent ischemic stroke during a mean follow up of 2.7 years. The 3–6 month MOCA score was performed on 351 patients. In prespecified multivariable models, there was an association between 3 and 6 month MOCA and recurrent stroke (hazard ratio [HR] per point increase .93 95% confidence interval [CI] .88–.99, P = .040). This effect was present in anterior circulation stenosis (adjusted HR per point increase .92 95% CI .85–0.99, P = .022) but not in posterior circulation artery stenosis (adjusted HR per point increase 1.00 95% .86–1.16, P = .983).
Conclusions:
Overall, we found weak associations and trends between MoCA at 3–6 months and stroke recurrence but more notable and stronger associations in certain subgroups. Since our study is underpowered, larger studies are needed to validate our findings and determine the mechanism (s) behind this association.
Keywords: Intracranial atherosclerosis, cognitive impairment, stroke, MOCA
Introduction
Cognitive impairment occurs in 20%–40% of stroke patients1–3 and is a predictor of long-term morbidity4 and mortality.5,6 The incidence of cognitive impairment after ischemic stroke is highest in patients with large vessel disease subtype,7 a finding that may be at least partially explained by the high risk of early recurrence and/or impaired distal impaired blood flow in some patients with extracranial or intracranial stenosis, particularly involving the anterior circulation.8–11 While impaired distal blood flow has been shown to predict stroke recurrence in patients with symptomatic intracranial stenosis,12 the association between poststroke cognitive impairment and risk of stroke recurrence remains unclear.
In this study, we aim to determine the association between poststroke cognitive impairment defined by the Montreal Cognitive Assessment (MOCA)13 (MOCA <22) at 3–6 months14,15 and stroke recurrence risk. Furthermore, given the lack of data on cognitive outcomes in relationship to blood flow in patients with arterial stenosis affecting the posterior circulation, we sought to stratify patients by location of the anterior stenosis (anterior versus posterior circulation).
Methods
Patient Cohort
We obtained approval from the National Institute of Neurological Disorders and Stroke to perform this analysis. The data is publicly available upon request from the National Institute of Neurological Disorders and Stroke. This is a post-hoc analysis of the Stenting and Aggressive Medical Therapy for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial that included patients with symptomatic intracranial stenosis causing 70% or more luminal narrowing and randomized them to aggressive medical treatment versus stenting plus aggressive medical treatment. Patients enrolled in SAMMPRIS were followed for up for a median of 32.4 months and the primary outcome was stroke or death within 30 days or ischemic stroke in the territory of the affected artery thereafter.
In this study, we included all patients enrolled in the SAMMPRIS trial except those who met the primary endpoint or secondary outcomes (asymptomatic intracranial hemorrhage or cerebral infarct with temporary symptoms) before they underwent neurocognitive testing at 3–6 months and those who did not undergo neurocognitive testing in the 3–6 months (+/− 5 days) interval after their index event.
Primary Predictor
Patients enrolled in SAMMPRIS were followed over time and certain clinical and laboratory tests were performed during follow up visits. Cognitive assessments using MOCA tool were performed at the following intervals: baseline, 4 months (performed at 3–6 months), 12 months, and close-out.16 Since cognitive testing at the time of an acute illness may be confounded by several factors and may not be solely related to the effect of the stroke, we chose the MOCA at the 3–6 month visit and not the baseline MOCA to be the primary predictor. This timing makes delirium less likely as a cause for a change in MOCA as delirium is more often transient in the setting of stroke.
Covariates
Study variables were abstracted from the publicly available SAMMPRIS dataset and included:
Baseline demographics: Age and sex.
Vascular risk factors: History of hypertension (known history of hypertension or on any treatment for hyper-tension), history of diabetes (known history of diabetes or on any treatment for diabetes), history of lipid disorder (known history of lipid disorder or on any treatment for lipid disorder), body mass index (weight/height2), smoking history (active or ex-smoker versus nonsmoker), physical activity in target range (PACE score >3),17 history of coronary artery disease (defined as coronary artery disease, myocar-dial infarction, angina, and coronary artery bypass surgery), history of congestive heart failure, history of carotid intervention, history of peripheral vascular disease (defined as previously treated for peripheral vascular disease), history of vascular disease (combination of coronary artery disease and peripheral vascular disease), history of ischemic stroke (not qualifying event), and stroke as qualifying event (versus transient ischemic attack).
Medications: Antithrombotic therapy at the time of enrollment and statin therapy at randomization.
Clinical variables: Systolic blood pressure at enrollment, diastolic blood pressure at enrollment.
Radiological variables: Degree of stenosis by central adjudication at baseline, anterior versus posterior circulation, and acute or subacute infarct in the territory of the affected artery.
Treatment assignment: Aggressive medical management versus stenting plus aggressive medical management.
Outcome
The outcome in this study was recurrent ischemic stroke in the territory of the affected artery occurring after the 3–6 months visit when the MOCA was performed. The recurrent ischemic stroke adjudication process in SAMMPRIS has been previously described.18
Statistical Analysis
Patients were divided into 2 groups – those with recurrent ischemic stroke versus those without recurrent ischemic stroke. We compared the covariates above, baseline MOCA, and MOCA scores at the 3–6 month visit between the 2 groups using Fisher’s exact test for categorical variables and t-test or nonparametric tests as indicated between continuous variables. To determine the association between MOCA and recurrent ischemic stroke, we performed a binary logistic regression analysis according to 2 models: model 1 (prespecified) including certain predictors of recurrent ischemic stroke in SAMMPRIS (stroke as qualifying event, statin at time of enrollment, and acute/subacute infarcts in the territory of the affected artery)19 and model 2 (hypothesis driven) included variables achieving statistical significance on univariate analyses (P< .05). We then performed the same analysis in patients with anterior circulation and in those with posterior circulation arterial territory and in those randomized to medical treatment versus those randomized to stenting. Analysis was done using SPSS version 25.0 (Chicago, IL) and P < .05 was considered statistically significant.
Results
Baseline Characteristics and Univariate Analyses
Of the 451 patients enrolled in SAMMPRIS, 393 patients met the inclusion criteria. Reasons for exclusion were 51 patients had the outcome of “stroke, symptomatic intracranial hemorrhage, or death” before the MOCA at the 3–6 month visit, 5 had cerebral infarction with temporary symptoms, and 2 asymptomatic hemorrhages occurring prior to the 3–6 month MOCA was performed. The mean age of the sample (in years) was 59.5 ± 11.3 and 62.6% (246 of 393) were men. For about 3–6 month MOCA score was performed on 351 patients at a range of 89 days to 185 days and 50 patients (12.7%) had recurrent ischemic stroke during a mean follow up of 2.7 years.
In univariate models, when compared to patients without recurrent ischemic stroke, patients with recurrent ischemic stroke were less likely to have hypertension (80% (40 of 50) versus 90.4% (310 of 343), P = .048), more likely to have diabetes (54% (27 of 50) versus 37.9% (130 of 343), P = .044), more likely to have a history of stroke (20 of 50 versus 77 of 343, P = .013) and less likely to be on a statin at the time of randomization (35 of 50 versus 302 of 343, P = .002). The mean MOCA at the 3–6 months visit was nonsignificantly lower in patients with versus without recurrent stroke (24.0 ± 5.0 versus 25.4 ± 4.1, P = .057). The mean MOCA at baseline was not significantly different between those with versus those without recurrent ischemic stroke (22.7 ± 6.3 versus 23.9 ± 4.6, P = .205). Other variables were not statistically significant and are listed in Table 1.
Table 1.
Baseline characteristics of patients with and without recurrent stroke
| Recurrent stroke after 90 days (n = 50) | No recurrent stroke after 90 days (n = 343) | P value | |
|---|---|---|---|
| Age (mean ± SD) | 59.6 ± 12.6 | 59.5 ± 11.1 | .957 |
| Sex (% women) | 40% (20) | 37.0% (127) | .755 |
| Body mass index (mean ± SD) | 29.6 ± 6.4 | 30.6 ± 6.2 | .272 |
| Hypertension | 80% (40) | 90.4% (310) | .048 |
| Diabetes | 54% (27) | 37.9% (130) | .044 |
| Hyperlipidemia | 80% (40) | 89.5% (307) | .060 |
| Ex-current smoking | 78% (39) | 63.9% (219) | .056 |
| In-target physical activity (PACE >3) | 24% (12) | 33.5% (115) | .197 |
| History of stroke | 40% (20) | 22.5% (77) | .013 |
| Coronary artery disease | 28% (14) | 21.9% (75) | .366 |
| Congestive heart failure | 6% (3) | 2.0% (7) | .122 |
| Qualifying event as stroke | 72% (36) | 63.3% (217) | .269 |
| Statin treatment at randomization | 70% (35) | 88.1% (302) | .002 |
| Antithrombotic at qualifying event | 70% (35) | 60.6% (208) | .217 |
| Systolic blood pressure (mean ± SD) | 145.9 ± 21.2 | 145.2 ± 21.3 | .862 |
| Diastolic blood pressure (mean ± SD) | 82.0 ± 11.3 | 80.6 ± 11.6 | .415 |
| Acute or subacute infarct in the territory | 64.6% (31/48) | 62.2% (207/333) | .874 |
| Randomized to medical treatment | 52% (26) | 53.4% (183) | .880 |
| Anterior circulation distribution | 70% (35) | 64.4% (221) | .526 |
| Percentage stenosis (mean ± SD) | 74.9 ± 9.5 | 74.2 ± 8.9 | .631 |
| MOCA at baseline (mean ± SD) | 22.7 ± 6.3 | 23.9 ± 4.6 | .205 |
| MOCA at 3–6 months (mean ± SD) | 24.0 ± 5.0 | 25.4 ± 4.1 | .057 |
| MOCA < 22 at 3–6 months | 23.1% (9/39) | 12.8% (40/312) | .089 |
Abbreviations: n, number of patient, SD, standard deviation; PACE, Patient-centered Assessment and Counseling for Exercise; MOCA, Montreal Cognitive Assessment
Association Between MOCA at 3–6 Months and Recurrent Stroke
In cox-regression models, there was a nonsignificant association between MOCA at 3–6 months and recurrent ischemic stroke in unadjusted (hazard ratio [HR] per point increase .94 95% confidence interval [CI] .88–1.00, P = .055) and predefined adjusted models (model 1) (HR per point increase .93 95% CI .88–.99, P = .040). The effect size was attenuated when adjusting for variables achieving significance on univariate analysis (model 2) (HR .96 95% CI .90–1.02, P = .200).
Association Between MOCA at 3–6 Months and Recurrent Stroke Based on Artery Location
The mean MOCA at 3–6 months was not significantly different between patients with anterior versus posterior circulation artery location (25.0 ± 4.5 versus 25.4 ± 3.9, P = .407). The association between MOCA at 3–6 months and recurrent stroke risk in model 1 was present in those with anterior circulation artery location (adjusted HR per point increase .92 95% CI .85–.99, P = .022) but not in posterior circulation artery location (adjusted HR per point increase 1.00 95% 0.86–1.16, p = .983; Table 2).
Table 2.
Cox-regression models showing association between 3 and 6 month MOCA and stroke recurrence
| Unadjusted (hazard ratio, 95% CI, P value) | Model 1 (hazard ratio, 95% CI, P value) | Model 2 (hazard ratio, 95% CI, P value) | |
|---|---|---|---|
| All patients | .94 (.88–1.00), p = .055 | 0.93 (.88–.99), p = .040 | 0.96 (.90–1.02), p = .200 |
| Anterior circulation | .92 (.86–0.99), p = .020 | 0.92 (.85–.99), p = .022 | 0.93 (.87–1.00), p = .063 |
| Posterior circulation | 1.01 (.88–1.17), p = .891 | 1.00 (.86–1.16) p = .983 | 1.04 (.89–1.21), p = .658 |
| Stenting arm | .95 (.86–1.04), p = .280 | 0.94 (.85–1.04), p = .224 | 0.96 (0.86–1.06) p = .394 |
| Medical arm | .93 (.86–1.01), p = .078 | 0.92 (.85–1.01), p = .078 | 0.95 (.87–1.04) p = .266 |
Model 1: adjusted for stroke as qualifying event, statin at time of enrolment, and infarct in the territory of affected artery; Model 2: adjusted for hypertension, diabetes, history of stroke, and statin at time of randomization.
Association Between MOCA at 3–6 Months and Recurrent Stroke Based on Treatment Assignment
The mean MOCA at 3–6 months was not significantly different between patients undergoing stenting versus those undergoing medical treatment (25.2 ± 4.1 versus 25.1 ± 4.4, P = .870). The effect of MOCA at 3–6 months in model 1 was not significantly different in patients assigned to medical treatment (adjusted HR per point increase .92 95% CI .85–1.01, P = .078) as compared to those patients randomized to stenting (adjusted HR per point increase .94 95% .85–1.04, P = .224; Table 2). The interaction effect for this association did not achieve statistical significance (P = .775).
Poststroke Dementia and Recurrent Ischemic Stroke
Based on previous literature to define poststroke dementia based on MOCA,14,15 we performed a post-hoc analysis dividing patients into 2 groups: 3–6 month MOCA <22 versus 3–6 month MOCA ≥22. In this analysis, there was a nonstatistically significant trend for association between MOCA <22 and recurrent ischemic stroke in all patients and those with anterior circulation lesions, but not those with posterior circulation lesions (Fig 1).
Figure 1.
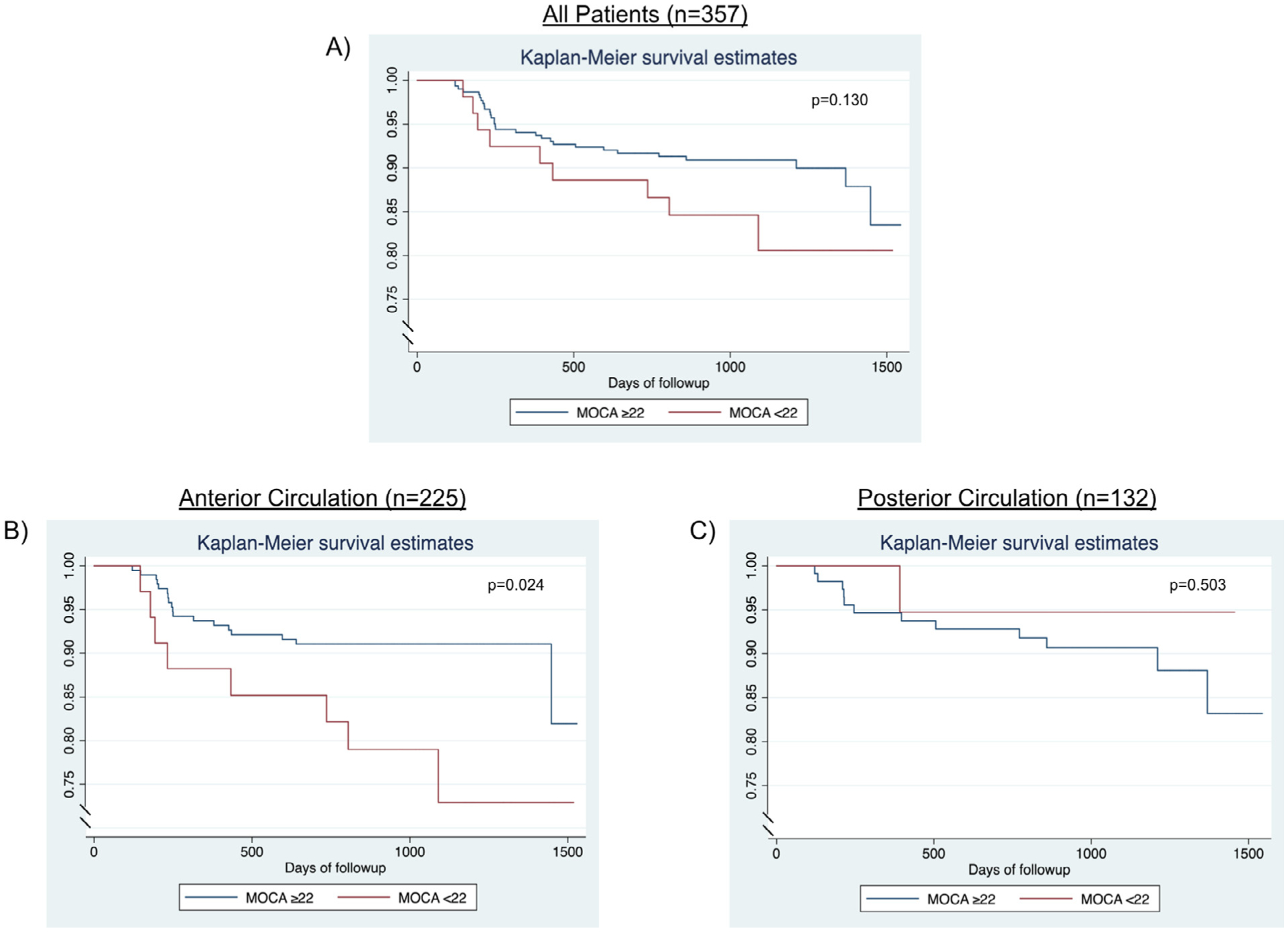
Kaplan-Meier survival estimates showing the association between a Montreal Cognitive Assessment Score (MOCA) of less than <22 and recurrent ischemic stroke risk: (A) all patient; (B) patients with anterior circulation arterial stenosis; (C) posterior circulation arterial stenosis.
Discussion
Study Findings
In this study, we found a relatively weak association between poststroke cognitive impairment (determined by MOCA at 3–6 months) and recurrent stroke risk in patients with symptomatic intracranial atherosclerosis enrolled in the SAMMPRIS trial. This association was more pronounced in patients with anterior circulation versus posterior circulation intracranial atherosclerotic disease. In addition, we found that the baseline poststroke MOCA was not associated with recurrent stroke.
Mechanisms of Associations
There are several potential mechanisms by which post-stroke cognitive impairment is associated with recurrent stroke risk. First, it is possible that our findings are explained by the fact that impaired cognition after a stroke may lead to a sedentary lifestyle, decreased physical activity, and reduced medication compliance in the setting of cognitive impairment, all of which may increase the risk of recurrent stroke. In fact, a post-hoc analysis of SAMMPRIS showed an association between out of target physical activity and impaired cognition20 and out of target physical activity during follow up and recurrent stroke.21 Second, it is possible that poststroke cognitive impairment may be a marker of severity of intracranial atherosclerotic disease and that these patients are more likely to have previous and more extensive infarcts leading to increased likelihood cognitive impairment. In fact, in the SAMMPRIS trial, stroke recurrence risk was more likely to occur in patients with prior infarcts19 which are associated with an increased likelihood of poststroke dementia.22 While we adjusted for prior ischemic stroke and acute/subacute infarcts in the territory of the affected artery, information on prior infarct in the same territory was not available in our dataset and therefore we are unable to prove or disprove this hypothesis. Third, it is also possible that the association between poststroke cognitive impairment and stroke recurrence is mediated by impaired blood flow. In patients with symptomatic anterior circulation intracranial atherosclerosis, studies have shown an association between perfusion delay,23 infarct pattern (borderzone versus nonborderzone),24,25 and stroke recurrence risk. This association was also shown in patients with symptomatic vertebrobasilar disease.26 On the other hand, studies have shown an association between impaired distal perfusion and cognition dysfunction.8,27 In our study, the association between cognitive impairment at 3–6-months and recurrent stroke had a trend to be more pronounced in an anterior circulation location. This may be explained by the fact that patients with posterior circulation involvement enrolled in SAMMPRIS generally had unilateral vertebral disease or basilar disease, a phenotype that is less likely to be associated with impaired distal blood flow as compared to those with tandem vertebrobasilar or bilateral vertebral artery lesions28 who were excluded from SAMMPRIS.18
Therapeutic Implications
This study has several clinical implications. First, it highlights the importance of cognitive testing in patients with symptomatic intracranial atherosclerosis, particularly in those with anterior circulation distribution as cognitive impairment could be a predictor of recurrent stroke. Thus, this subset of patients may be a target for more frequent follow-up and potentially more aggressive secondary stroke prevention strategies, particularly physical activity that may lead to improved cognition and a reduction in stroke recurrence risk. Health care providers face many challenges in implementing stroke prevention strategies in patients with poststroke cognitive impairment.29 Tools such as medication management programs,30 exercise programs tailored to those with impaired cognition,31 patient/family education,30 and home health services may help improve compliance with these strategies and reduce the recurrence risk in this patient population. In addition, studies are needed to investigate the role of medications associated with improved memory such as donepezil and memantine and improved attention such as modafinil to see if they lead to improved poststroke cognition and reduced recurrent stroke risk.
Second, if impaired blood flow contributes to cognitive dysfunction and stroke recurrence risk in patients with symptomatic intracranial atherosclerosis, establishing blood flow criteria that would define these phenotypes may be an important next step in determining a subset of patients where reperfusion therapies may lead to a reduced stroke recurrence risk and perhaps improved cognition. In fact, in patients with asymptomatic severe extracranial carotid stenosis, the CREST-H trial study is investigating whether carotid revascularization improves cognition in these patients.32 This will shed light on whether revascularization techniques in general provide improve cognitive outcomes in patients with impaired perfusion.
Strengths and Limitations
Our study has several limitations, including its retrospective nature and a number of patients who lost to follow up and ineligible due to no MOCA available. Moreover, since this study is based off the SAMMPRIS trial, we are biased by the inclusion/exclusion of SAMMPRIS, most importantly our patient population is relatively young (mean age 59.5 years). In addition, we lack data on perfusion imaging, infarct pattern, and infarct burden could be helpful to explain the associations found in our study. We also lack data on poststroke depression and sleep apnea which may alter cognitive function and increase stroke risk. These should be explored in future studies. In addition, we did not account for medication such as memantine and donepezil use that may affect cognitive function. Moreover, we lack data on the extent of sensory and motor deficits during follow-up, as sensory and motor limitations may have arti-ficially impacted both MOCA performance and other variables (e.g., mobility/physical activity capacity) that may covary with recurrence risk. Furthermore, the MOCA was included was between 89 and 185 days which is a relatively wide range. Most of the stroke recovery, however, occurs in the first 3 months and therefore this variability is not likely to be a major source of bias. In addition, due to the relatively small number of events, we are likely underpowered to determine association between MOCA at 3–6 months and recurrent ischemic stroke risk. Another major limitation is that we used MOCA to assess cognitive function which is a limited cognitive assessment tool when compared to a more comprehensive neuropsychological evaluation, which should be explored in future studies.
On the other hand, our study has several strengths including analyzing randomized controlled trial data, blinded outcome adjudication, and being multicenter which makes it more generalizable.
Conclusions
Overall, we found weak associations and trends between MoCA at 3–6 months and stroke recurrence but more notable and stronger associations in certain subgroups. Since our study is underpowered, larger studies are needed to validate our findings and determine the mechanism(s) behind this association. Since our study is underpowered, larger studies are needed to validate our findings and determine the mechanism(s) behind this association.
Financial disclosures:
SAMMPRIS was funded by the National Institute of Neurological Disorders and Stroke (grant no. U01 NS058728).
Footnotes
Disclosures
The authors report no significant disclosures.
References
- 1.Desmond DW, Moroney JT, Sano M, et al. Incidence of dementia after ischemic stroke: results of a longitudinal study. Stroke 2002;33:2254–2260. [DOI] [PubMed] [Google Scholar]
- 2.Tatemichi TK, Paik M, Bagiella E, et al. Risk of dementia after stroke in a hospitalized cohort: results of a longitudinal study. Neurology 1994;44:1885–1891. [DOI] [PubMed] [Google Scholar]
- 3.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA 2015;314:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desmond DW, Moroney JT, Bagiella E, et al. Dementia as a predictor of adverse outcomes following stroke: an evaluation of diagnostic methods. Stroke 1998;29:69–74. [DOI] [PubMed] [Google Scholar]
- 5.Tatemichi TK, Paik M, Bagiella E, et al. Dementia after stroke is a predictor of long-term survival. Stroke 1994;25:1915–1919. [DOI] [PubMed] [Google Scholar]
- 6.Desmond DW, Moroney JT, Sano M, et al. Mortality in patients with dementia after ischemic stroke. Neurology 2002;59:537–543. [DOI] [PubMed] [Google Scholar]
- 7.Levine DA, Wadley VG, Langa KM, et al. Risk factors for poststroke cognitive decline: The regards study (reasons for geographic and racial differences in stroke). Stroke 2018;49:987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leeuwis AE, Smith LA, Melbourne A, et al. Cerebral blood flow and cognitive functioning in a community-based, multi-ethnic cohort: the sabre study. Front Aging Neurosci 2018;10:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marshall RS, Festa JR, Cheung YK, et al. Cerebral hemodynamics and cognitive impairment: baseline data from the recon trial. Neurology 2012;78:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marshall RS, Lazar RM. Pumps, aqueducts, and drought management: vascular physiology in vascular cognitive impairment. Stroke 2011;42:221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norling AM, Marshall RS, Pavol MA, et al. Is hemispheric hypoperfusion a treatable cause of cognitive impairment. Curr Cardiol Rep 2019;21:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaghi S, Prabhakaran S, Khatri P, et al. Intracranial atherosclerotic disease. Stroke 2019;50:1286–1293. [DOI] [PubMed] [Google Scholar]
- 13.Nasreddine ZS, Phillips NA, Bedirian V, et al. The montreal cognitive assessment, MOCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 14.Lees R, Selvarajah J, Fenton C, et al. Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 2014;45:3008–3018. [DOI] [PubMed] [Google Scholar]
- 15.Dong Y, Sharma VK, Chan BP, et al. The Montreal cognitive assessment (MOCA) is superior to the mini-mental state examination (mmse) for the detection of vascular cognitive impairment after acute stroke. J Neurol Sci 2010;299:15–18. [DOI] [PubMed] [Google Scholar]
- 16.Turan TN, Smock A, Cotsonis G, et al. Is there benefit from stenting on cognitive function in intracranial atherosclerosis. Cerebrovasc Dis (Basel, Switzerland) 2017;43:31–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan GS, Haase AM, Campbell R, et al. Physical activity facilitation for elders (pace): study protocol for a rand-omised controlled trial. Trials 2015;16:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chimowitz MI, Lynn MJ, Turan TN, et al. Design of the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. J Stroke Cerebrovasc Dis 2011;20:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters MF, Hoh BL, Lynn MJ, et al. Factors associated with recurrent ischemic stroke in the medical group of the sammpris trial. JAMA Neurol 2016;73:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turan TN, Al Kasab S, Smock A, et al. Impact of baseline features and risk factor control on cognitive function in the stenting and aggressive medical management for preventing recurrent stroke in intracranial stenosis trial. Cerebrovasc Dis (Basel, Switzerland) 2019;47:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turan TN, Nizam A, Lynn MJ, et al. Relationship between risk factor control and vascular events in the sammpris trial. Neurology 2017;88:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet. Neurology 2009;8:1006–1018. [DOI] [PubMed] [Google Scholar]
- 23.Yaghi S, Khatri P, Prabhakaran S, et al. What threshold defines penumbral brain tissue in patients with symptomatic anterior circulation intracranial stenosis: an exploratory analysis. J Neuroimaging 2019;29:203–205. [DOI] [PubMed] [Google Scholar]
- 24.Wabnitz AM, Derdeyn CP, Fiorella DJ, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke 2019;50:143–147. Strokeaha118020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yaghi S, Grory BM, Prabhakaran S, et al. Infarct pattern, perfusion mismatch thresholds, and recurrent cerebrovascular events in symptomatic intracranial stenosis. J Neuroimaging 2019;29:640–644. [DOI] [PubMed] [Google Scholar]
- 26.Amin-Hanjani S, Pandey DK, Rose-Finnell L, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol 2016;73:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Y, Wang L, Sun X, et al. Association between cerebral hypoperfusion and cognitive impairment in patients with chronic vertebra-basilar stenosis. Front Psychiatry 2018;9:455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amin-Hanjani S, Du X, Rose-Finnell L, et al. Hemodynamic features of symptomatic vertebrobasilar disease. Stroke 2015;46:1850–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jamison J, Sutton S, Mant J, et al. Barriers and facilitators to adherence to secondary stroke prevention medications after stroke: analysis of survivors and caregivers views from an online stroke forum. BMJ Open 2017;7: e016814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yetzer E, Blake K, Goetsch N, et al. Safe medication management for patients with physical impairments of stroke, part two. Rehabil Nurs 2017;42:282–289. [DOI] [PubMed] [Google Scholar]
- 31.Littbrand H, Rosendahl E, Lindelof N, et al. A high-intensity functional weight-bearing exercise program for older people dependent in activities of daily living and living in residential care facilities: evaluation of the applicability with focus on cognitive function. Physical Ther 2006;86:489–498. [PubMed] [Google Scholar]
- 32.Marshall RS, Lazar RM, Liebeskind DS, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis - hemodynamics (crest-h): study design and rationale. Int J Stroke 2018;13:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]


