Figure 6.
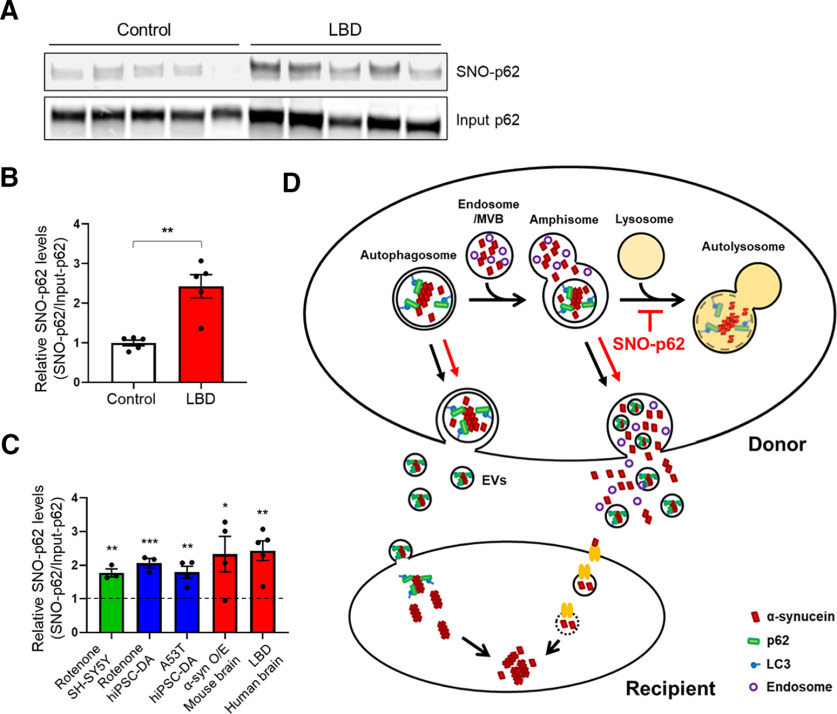
S-Nitrosylation of p62 in human LBD brains and schema for α-syn cell-to-cell spread. A, S-Nitrosylation of human LBD and control brains. Human brain tissues with relatively short postmortem times (described in Extended Data Fig. 6-1) were subjected to biotin-switch assay. B, Ratio of SNO-p62/input p62 from control or LBD brains. Data are mean ± SEM; n = 5 brains in each group. **p < 0.01, Student's t test. C, Combined data for ratio of SNO-p62/Input p62 from Figures 1D, F, H and 6B (asterisks are from the original figures and retain their original meaning; vertical dotted line at “1” indicates the control for each condition from data shown in each of the other figures listed above). D, Schema of mechanism for S-nitrosylated p62 increasing cell-to-cell transmission of α-syn. Cytoplasmic misfolded α-syn can be directly released via nonclassical exocytosis or exophagy, yielding free α-syn in the extracellular medium (Ejlerskov et al., 2013). Alternatively, misfolded α-syn can be relegated to multivesicular bodies in the cell and then released into the extracellular space via enriched exosomes or EVs (Jang et al., 2010; Alvarez-Erviti et al., 2011; Danzer et al., 2012; Lee et al., 2013). SNO-p62 enhances the release of misfolded α-syn via both of these pathways by enhancing protein buildup because of blockade of autophagy at the stage of autolysosome formation. Released α-syn is then internalized by neighboring cells, and can seed endogenous α-syn to form aggregates (Luk et al., 2009; Nonaka et al., 2010). EV-mediated uptake occurs through direct endocytosis of the vesicle (Lee et al., 2008), while EV-independent uptake of α-syn may be mediated by binding to a receptor on the cell surface of the cell with subsequent endocytosis and endosome formation of the complex (Mao et al., 2016). See Extended Data Figure 6-1.