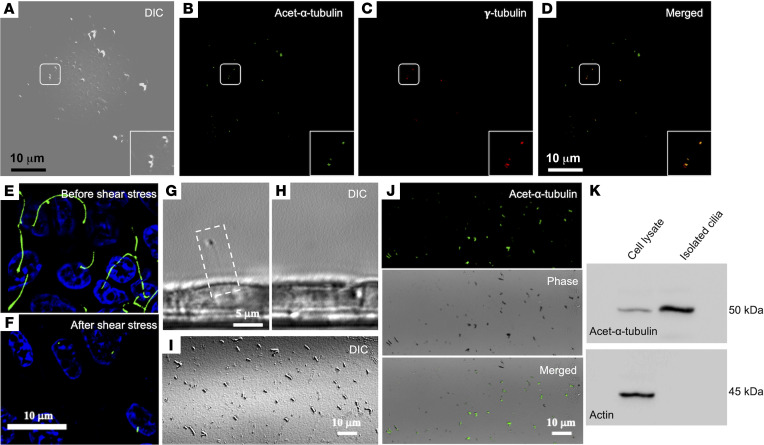
Figure 2. Shear stress causes deciliation of endothelial and epithelial cells in vitro.
(A–D) HUVECs grown on monolayer. Fluid shear stress of 20 dyne/cm2 was perfused onto the cells, and the perfusate was collected. The drop concentrated perfusate was analyzed with DIC microscopy and stained with markers for cilia (acetylated-α-tubulin) (B) and basal body (γ-tubulin) (C). Enlarged images are also shown in the boxes. (D) Merged image is shown. (E and F) Immunostaining (acetylated-α-tubulin, green, cilia; and DAPI, blue, nucleus) for the presence and absence of the cilia from the epithelial cell population before (upper panel) and after (lower panel) the application of 10 dyne/cm2 shear stress, respectively. (G and H) Phase contrast DIC image of a primary cilium in a single live cell (white dotted box). The same cell was imaged before and after 4-minute application of 10 dyne/cm2 fluid shear stress. (I) Perfusate under DIC microscopy. (J) A separate experiment, where perfusate was collected and stained with ciliary marker (acetylated-α-tubulin; green) to confirm the presence of cilia using both fluorescence and phase contrast imaging (I). (K) Cilia and cell lysates were immunoblotted with cilia marker (acetylated-α-tubulin) to molecularly confirm the presence of the cilia in the perfusate (n = 6). E–K represent porcine kidney epithelial cells (LLC-PK1).