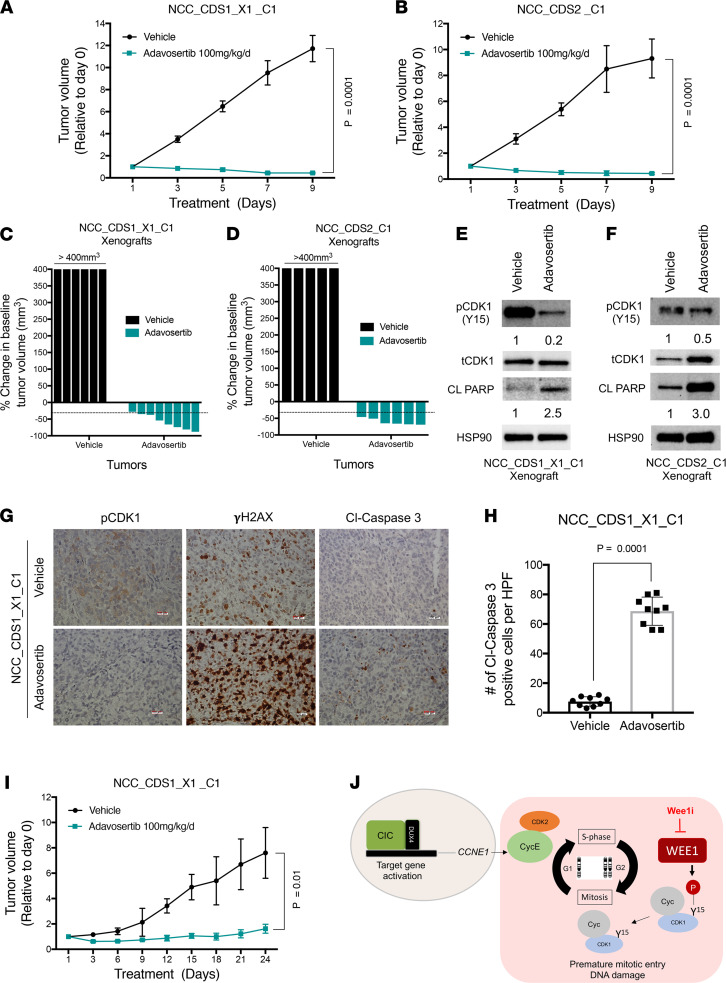
Figure 4. WEE1 is a therapeutic target in CIC-DUX4 sarcomas.
Relative tumor volume of NCC_CDS1_X1_C1 (A) (n = 8 for adavosertib and n = 6 for vehicle control groups) and NCC_CDS2_C1 (B) (n = 6 for adavosertib and n = 5 for vehicle control groups) treated with adavosertib or vehicle control. Student’s t test. Error bars represent SEM. (C and D) Percent change from baseline in tumor volume for NCC_CDS1_X1_C1 (n = 8 for adavosertib and n = 6 for vehicle control group) and NCC_CDS2_C1 (n = 6 for adavosertib and n = 5 for vehicle control) tumor–bearing mice in the adavosertib and vehicle cohorts. Percent change from baseline tumor volume for each mouse is shown in Supplemental Figure 5, C and D. (E and F) Immunoblot of NCC_CDS1_X1_C1 and NCC_CDS2_C1 tumor explants treated with adavosertib or vehicle control. (G) Representative IHC images of pCDK1, γH2AX, and cleaved caspase-3 in NCC_CDS1_X1_C1 tumor xenografts derived from mice treated with adavosertib or vehicle control. Scale bars: 100 μm. (H) Number of cleaved caspase-3–positive cells per HPF; 9 HPFs analyzed. Student’s t test. (I) Tumor volume of NCC_CDS1_X1_C1 xenograft–bearing mice treated for 24 days with adavosertib (n = 6) compared with vehicle control (n = 6). (J) Model: CIC-DUX4–regulated CCNE1 transcriptional upregulation leads to survival dependence on the G2/M checkpoint kinase WEE1. Wee1i, WEE1 inhibition.