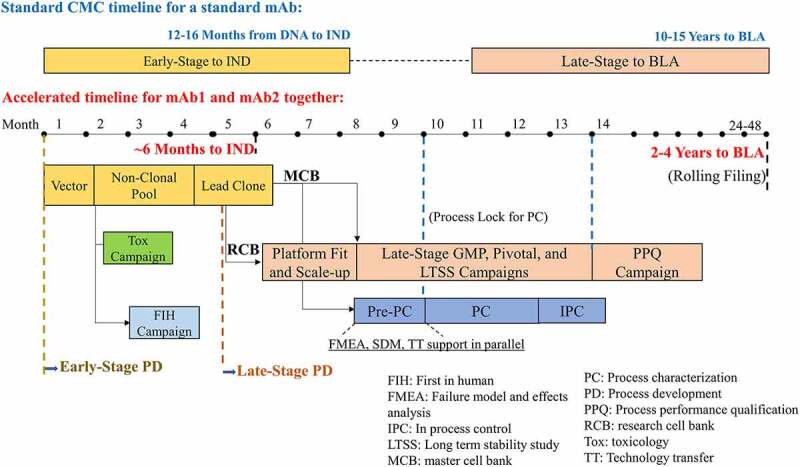
Figure 1.
Standard mAb CMC timeline versus the accelerated development timeline of mAb1 and mAb2 together for early-stage CMC development towards IND filing and late-stage CMC development towards BLA submission.Non-clonal pools were used to generate Tox study and FIH Phase 1 study materials simultaneously. The early-stage timeline from DNA to IND was shortened from 12-16 to 6 months for the accelerated timeline. Late-stage process development including scale-up run was accelerated by using RCB and platform fit. The start of late-stage GMP manufacturing was accelerated using MCB and the platform process before the final process lock. Then formal PC studies and pivotal GMP campaign were started right after the process lock. Overall accelerated timeline from DNA to BLA would be shortened from a standard of 10-15 years to 2-4 years.
Alt Text: Monthly process development activities for mAb1 and mAb2 from a vector construction to IND submission for early-stage and from platform fit lab runs to the PPQ campaign for late stage. The early-stage timeline from DNA to IND was shortened from 12–16 to 6 months for mAb1 and mAb2 using the accelerated timeline, while overall accelerated timeline from DNA to BLA would be shortened from 10–15 to 2–4 years.