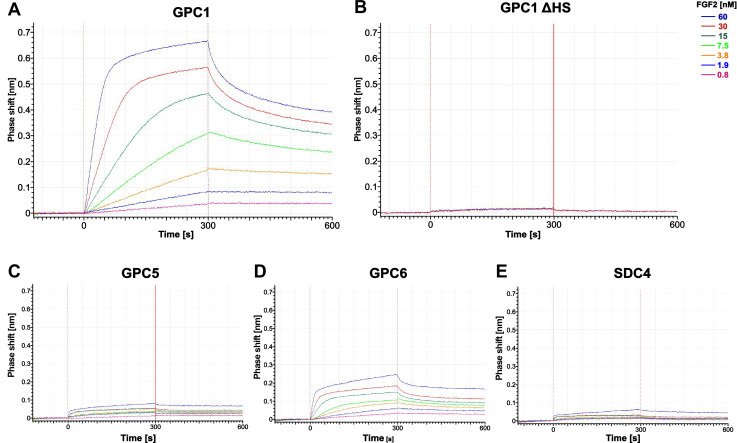
Figure 6. Glypican-1 (GPC1) and fibroblast growth factor 2 (FGF2) form a strong pair of interaction partners.
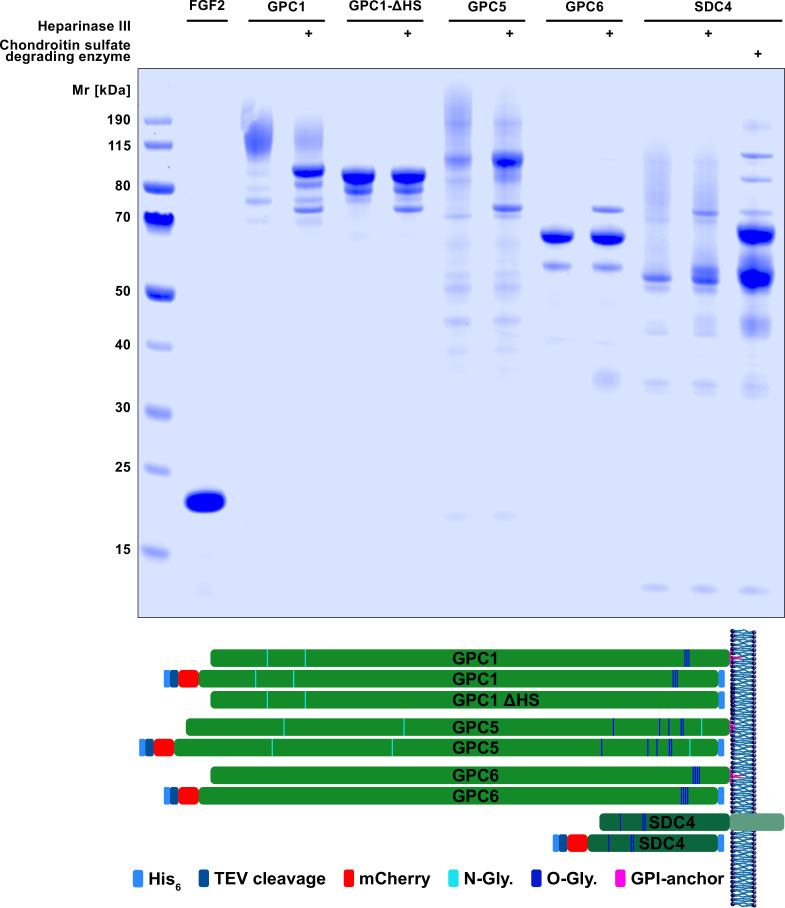
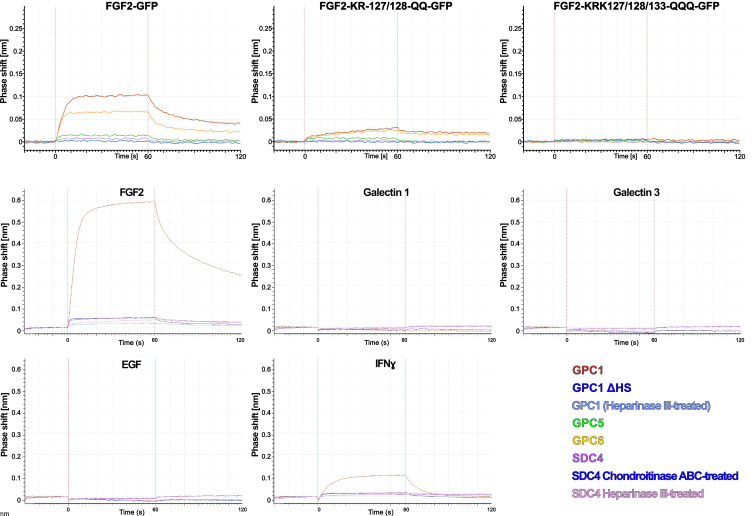
Recombinant constructs encoding soluble ectodomains of GPC1 (panel A), GPC1 ΔHS (panel B; a mutant form to which heparan sulfate chains cannot be added), GPC5 (panel C), GPC6 (panel D), and SDC4 (panel E; a member of the syndecan family of heparan sulfate proteoglycans) were expressed and purified from HEK293 cells (see Figure 6—figure supplement 1). Using biolayer interferometry, interactions studies with temporal resolution visualizing both association and dissociation kinetics were conducted with purified FGF2 (Figure 6—figure supplement 1) at the concentrations indicated. The data shown are representative for two independent experiments. Experimental details are given in the Materials and methods section.