Abstract
Aims
Hypertriglyceridaemia is associated with increased risk of cardiovascular events. This clinical trial evaluated olezarsen, an N-acetyl-galactosamine-conjugated antisense oligonucleotide targeted to hepatic APOC3 mRNA to inhibit apolipoprotein C-III (apoC-III) production, in lowering triglyceride levels in patients at high risk for or with established cardiovascular disease.
Methods and results
A randomized, double-blind, placebo-controlled, dose-ranging study was conducted in 114 patients with fasting serum triglycerides 200–500 mg/dL (2.26–5.65 mmol/L). Patients received olezarsen (10 or 50 mg every 4 weeks, 15 mg every 2 weeks, or 10 mg every week) or saline placebo subcutaneously for 6–12 months. The primary endpoint was the percent change in fasting triglyceride levels from baseline to Month 6 of exposure. Baseline median (interquartile range) fasting triglyceride levels were 262 (222–329) mg/dL [2.96 (2.51–3.71) mmol/L]. Treatment with olezarsen resulted in mean percent triglyceride reductions of 23% with 10 mg every 4 weeks, 56% with 15 mg every 2 weeks, 60% with 10 mg every week, and 60% with 50 mg every 4 weeks, compared with increase by 6% for the pooled placebo group (P-values ranged from 0.0042 to <0.0001 compared with placebo). Significant decreases in apoC-III, very low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B were also observed. There were no platelet count, liver, or renal function changes in any of the olezarsen groups. The most common adverse event was mild erythema at the injection site.
Conclusion
Olezarsen significantly reduced apoC-III, triglycerides, and atherogenic lipoproteins in patients with moderate hypertriglyceridaemia and at high risk for or with established cardiovascular disease.
Trial registration number
Keywords: Atherosclerosis, Cardiovascular disease, Cardiovascular risk factors, Hypertriglyceridaemia, apoC-III, Antisense
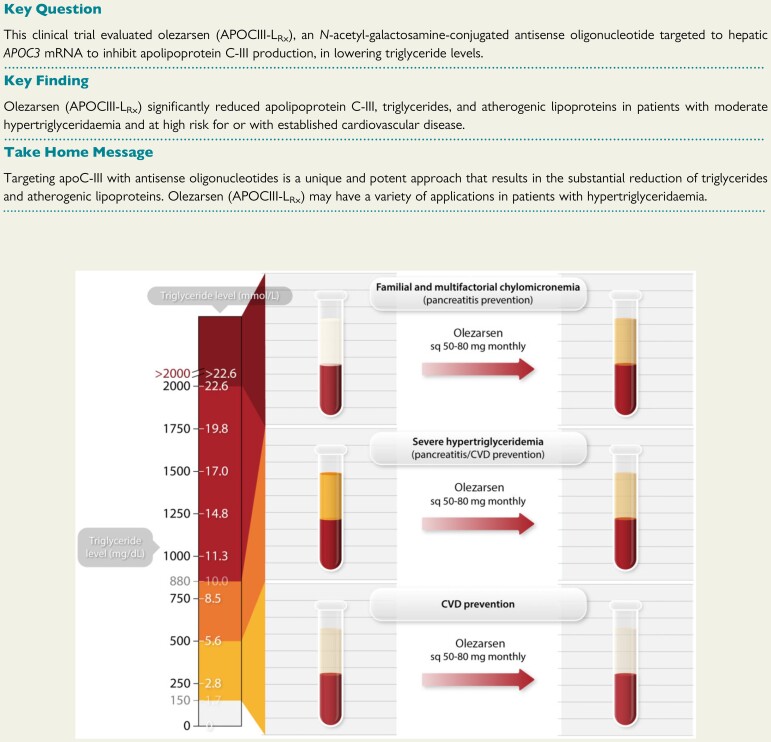
Structured Graphical Abstract
Structured Graphical Abstract.
Potential clinical indications for olezarsen. Triglyceride levels represent a continuum of risk with levels 1.7–5.6 mmol/L (150–500 mg/dL) representing primarily cardiovascular disease risk (cardiovascular disease prevention), levels between 5.6 and 10.0 mmol/L (500–885 mg/dL) representing both cardiovascular disease and pancreatitis risk and >10.0 mmol/L primarily pancreatitis risk in patients with familial chylomicronemia syndrome and multifactorial chylomicronemia syndrome (represented by milky plasma). Treatment with olezarsen with the planned Phase 3 doses of 50 and 80 mg subcutaneously monthly would be expected to substantially reduce triglyceride levels in the entire continuum of hypertriglyceridaemia.
See the editorial comment for this article ‘Apolipoprotein C-III inhibition to lower triglycerides: one ring to rule them all?’, by Robert A. Hegele, https://doi.org/10.1093/eurheartj/ehab890.
Introduction
Elevated levels of triglycerides and triglyceride-rich lipoproteins (TRL) are consistently associated with higher risk for atherosclerotic cardiovascular disease (ASCVD) in epidemiological, genetic, and clinical studies.1–3 However, their direct contribution to ASCVD to date remains unproven, as lowering of triglyceride levels has not been conclusively shown to reduce risk of ASCVD.4–6 Elevated triglycerides are strongly associated with elevated levels of TRLs that contain significant amounts of remnant cholesterol not addressable by low-density lipoprotein cholesterol (LDL-C)-directed therapies.7 , 8 Inadequately lowering TRLs with current lipid-lowering therapies may fail to fully manage lipid-related ASCVD risk.
Apolipoprotein C-III (apoC-III) is a key regulator of plasma triglyceride levels by modulating TRL hepatic uptake through lipoprotein lipase (LPL)-dependent and LPL-independent mechanisms.9 , 10 It has been suggested that apoC-III may exert proatherogenic effects directly by enhancing vessel wall inflammation and indirectly by promoting hypertriglyceridaemia.11 Individuals with loss of function mutations in APOC3 show ∼40% reduction in both triglyceride levels and risk for ASCVD compared with noncarriers.12–14 Furthermore, epidemiological studies have concluded that apoC-III levels predict risk of ASCVD and cardiovascular mortality.15–17
Olezarsen (also known as ISIS 678354; AKCEA-APOCIII-LRx) is an N-acetyl-galactosamine (GalNAc)-conjugated, antisense oligonucleotide targeted to hepatic APOC3 mRNA to inhibit apoC-III protein production.18 Olezarsen has been shown to decrease levels of serum triglycerides and TRL in healthy individuals with modestly elevated triglyceride levels, but its effect on higher risk populations has not been determined.
The present study was designed to evaluate the effect of apoC-III inhibition on levels of triglycerides and TRLs in patients with triglyceride levels 200–500 mg/dL (2.26–5.65 mmol/L) and at high risk for or with established ASCVD. Olezarsen was designed to provide enhanced safety and tolerability through the advantages of a lower dose and volume injection with less frequent dosing compared with volanesorsen, an unconjugated apoC-III inhibitor.10 , 19–21
Methods
Trial design
We conducted this Phase 2, dose-ranging, randomized, double-blind, placebo-controlled trial evaluating Olezarsen at 41 sites in the USA and Canada. Patients were randomized to one of the four treatment groups. Within each group, randomization was performed in a 4:1 ratio (Olezarsen:placebo) (Supplementary material online, Figure S1). The patients in each group were given one of four treatment regimens, with Olezarsen or placebo subcutaneously for a minimum of 6 months: Olezarsen at dose of 10 mg every 4 weeks, 15 mg every 2 weeks, 10 mg every week, and 50 mg every 4 weeks (equivalent to monthly dose of 10, 30, 40, or 50 mg) or saline placebo. The timing of the primary efficacy endpoint analysis was at Week 25 for patients who received every 4-week dosing, and at Week 27 for patients who received every 2-week or weekly dosing to achieve 6 months of exposure for each group.
The treatment portion of the study was continued up to 12 months or until the last randomized patient reached 6 months of exposure to collect additional long-term safety and efficacy data. A post-treatment follow-up period lasted for 13 weeks. The platelet count was monitored every 2 weeks simultaneously by central and local laboratories, and renal and liver function were monitored every 2 weeks for the first 3 months and monthly thereafter throughout the treatment period. Monitoring and stopping rules related to platelet count, renal function, and liver function were pre-specified in the protocol, including threshold limits (Supplementary material online). An independent Data and Safety Monitoring Board oversaw the study.
Akcea Therapeutics sponsored the trial and was responsible for its conduct and oversight, the collection and management of the data, the statistical analyses, and, along with the first author, data interpretation. The protocol was approved by the relevant health authorities, institutional review boards, and ethics committees.
Eligibility
Patients who were 18–80 years of age and had established ASCVD [documented coronary artery disease (CAD), stroke, or peripheral artery disease] or were at high risk for ASCVD defined as having Type 2 diabetes mellitus requiring treatment, age ≥50 years, and one additional ASCVD risk factor (see definition in the Supplementary material online), and had an elevated screening fasting triglyceride level [triglyceride ≥ 200 mg/dL (2.26 mmol/L) and ≤ 500 mg/dL (5.65 mmol/L)] were eligible for enrolment. Patients had to be on standard-of-care preventative therapy for their cardiovascular disease (CVD) risk factors, and those who were being treated with lipid-lowering medications, or hormones affecting lipid control could be enrolled if they had been on a stable drug regimen for 4 weeks before screening and were expected to remain on that regimen during the trial. Exclusion criteria included acute coronary syndrome, major cardiac surgery, or stroke or transient ischaemic attack within 6 months before screening; coronary, carotid, or peripheral revascularization, major non-cardiac surgery, or lipoprotein apheresis within 3 months before screening; heart failure of New York Heart Association Class IV; uncontrolled hypertension (systolic blood pressure >160 mmHg, or diastolic blood pressure >100 mmHg); Type 1 or Type 2 diabetes mellitus if newly diagnosed, or with change in anti-diabetic pharmacotherapy within 12 weeks of screening or expected need for change during the trial, use of glucagon-like peptide-1 agonists in patients with history of pancreatitis, or glycated haemoglobin ≥9.0%; an estimated glomerular filtration rate of <60 mL/min; a ratio of urine protein to creatinine of ≥250 mg/g; an alanine aminotransferase or aspartate aminotransferase level exceeding twice the upper limit of the normal range; total bilirubin level exceeding 1.2 times the upper limit of the normal range; a platelet count lower than the lower limit of the normal range; LDL-C > 130 mg/dL (>3.4 mmol/L); and use of anticoagulant drugs. Additional details are provided in the Supplementary material online. All trial participants provided written informed consent.
Laboratory measurements
All laboratory samples (blood and urine) were measured with commercially available assays at MedPace Reference Laboratories. For measurement of lipid parameters, fasted blood samples were collected. LDL-C was measured by ultracentrifugation. Very low-density lipoprotein cholesterol (VLDL-C) was calculated as: total cholesterol – [LDL-C + high-density lipoprotein cholesterol (HDL-C)] or calculated as triglyceride level divided by 5 when LDL-C values were not available in two patients (one treated with placebo and one treated with Olezarsen 50 mg every 4 weeks).
Endpoints
The primary efficacy endpoint was the percent change in fasting triglyceride levels from baseline to the primary analysis time point at 6 months of exposure (Week 25 or 27) in each olezarsen group as compared with the pooled placebo group. The unranked secondary end points were analysed in the same way. The secondary endpoints were the percent change from baseline in apoC-III, total cholesterol, LDL-C, HDL-C, non-HDL-C, VLDL-C, apoB, and apoA-I; proportion of patients who achieved serum triglyceride levels < 150 mg/dL (< 1.7 mmol/L) or triglyceride levels < 100 mg/dL (< 1.13 mmol/L). Exploratory endpoints were the percent change from baseline in lipoprotein(a) and angiopoietin-like 3 (ANGPTL3).
Statistical analysis
The power calculations are shown in the Supplementary material online. All efficacy analyses were performed with the full analysis set, defined as all patients who had undergone randomization and had received at least one dose of olezarsen or placebo. For the primary endpoint of percent change from baseline in triglyceride level, pairwise comparison between each olezarsen group and pooled placebo group was performed using an analysis of covariance (ANCOVA) model including treatment group as a fixed factor and log-transformed baseline as a covariate. Missing data for the primary efficacy endpoint were handled by a multiple imputation model that contained the following variables: log-transformed baseline fasting triglyceride value, log-transformed fasting post-baseline triglyceride values, stratified by treatment. The imputations were performed for post-baseline values by the Markov chain Monte Carlo method (MCMC). For the secondary endpoints (percent change from baseline), a similar ANCOVA model was performed without applying any imputation for missing data. For proportion of patients achieving serum triglyceride levels <150 mg/dL (<1.7 mmol/L), or triglyceride <100 mg/dL (<1.13 mmol/L), logistic regression was performed with MCMC imputation. Because of the exploratory nature of this Phase 2 trial, the P-values and widths of the 95% confidence intervals were not adjusted for multiplicity. All safety analyses were performed in the safety population, defined as all patients who underwent randomization and received at least one dose of olezarsen or placebo.
Results
Patients
In total, 449 patients were screened, of whom 114 were randomized to receive either olezarsen or placebo (Supplementary material online, Figure S2 and Supplementary material online, Table S1). The first patient was enrolled on 20 February 2018, and the last patient was enrolled on 17 May 2019. For the pooled olezarsen and placebo groups, the mean (±SD) duration of treatment was 244 ± 102 days (median, 232) and 215 ± 107 days (median, 200.5), and mean compliance (percent of total scheduled volume of drug received) was 96% and 94%, respectively.
Demographics and baseline characteristics of patients were generally similar across treatment groups (Table 1): 60.5% of patients were 65 years of age or older, and 75.4% were male. By protocol design, all patients had either established ASCVD (79.8%) or were at high risk for ASCVD (20.2%). The majority of patients (74.6%) had documented CAD. The most common CV risk factors included: hypertension (88.6%), history of hypercholesterolaemia (72.8%), smoking (ever 69.3%, current 14.9%), and Type 2 diabetes mellitus (67.5%).
Table 1.
Demographic, clinical, and laboratory characteristics at baseline olezarsen
| Baseline characteristics | Pooled placebo | Olezarsen | All patients (n = 114) | ||||
|---|---|---|---|---|---|---|---|
| (n = 24) | 10 mg | 15 mg | 10 mg | 50 mg | Pooled | ||
| Every 4 weeks | Every 2 weeks | Every week | Every 4 weeks | (n = 90) | |||
| (n = 22) | (n = 23) | (n = 23) | (n = 22) | ||||
| Age, years | 64.6 ± 7.93 | 64.4 ± 9.13 | 68.9 ± 6.82 | 65.6 ± 8.48 | 62.9 ± 7.40 | 65.5 ± 8.17 | 65.3 ± 8.10 |
| Age ≥65 years, n (%) | 13 (54.2) | 13 (59.1) | 19 (82.6) | 14 (60.9) | 10 (45.5) | 56 (62.2) | 69 (60.5) |
| Male sex, n (%) | 20 (83.3) | 14 (63.6) | 19 (82.6) | 18 (78.3) | 15 (68.2) | 66 (73.3) | 86 (75.4) |
| White race, n (%) | 22 (91.7) | 20 (90.9) | 23 (100) | 23 (100) | 18 (81.8) | 84 (93.3) | 106 (93.0) |
| BMI, kg/m2 | 32.1 ± 4.18 | 32.2 ± 3.92 | 31.5 ± 3.60 | 31.9 ± 4.45 | 32.8 ± 4.14 | 32.1 ± 4.00 | 32.1 ± 4.02 |
| CV risk factors, n (%) | |||||||
| Hypertension | 23 (95.8) | 20 (90.9) | 20 (87.0) | 21 (91.3) | 17 (77.3) | 78 (86.7) | 101 (88.6) |
| Hypercholesterolaemia | 19 (79.2) | 16 (72.7) | 18 (78.3) | 13 (56.5) | 17 (77.3) | 64 (71.1) | 83 (72.8) |
| Type 2 diabetes | 17 (70.8) | 17 (77.3) | 17 (73.9) | 14 (60.9) | 12 (54.5) | 60 (66.7) | 77 (67.5) |
| Family history of CAD | 9 (37.5) | 9 (40.9) | 13 (56.5) | 12 (52.2) | 15 (68.2) | 49 (54.4) | 58 (50.9) |
| Ever smoker | 19 (79.2) | 14 (63.6) | 14 (60.9) | 18 (78.3) | 14 (63.6) | 60 (66.7) | 79 (69.3) |
| Current smoker | 4 (16.7) | 6 (27.3) | 2 (8.7) | 1 (4.3) | 4 (18.2) | 13 (14.4) | 17 (14.9) |
| Coronary artery disease, n (%) | 20 (83.3) | 10 (45.5) | 18 (78.3) | 18 (78.3) | 19 (86.4) | 65 (72.2) | 85 (74.6) |
| Coronary revascularization | 14 (58.3) | 9 (40.9) | 15 (65.2) | 15 (65.2) | 14 (63.6) | 53 (58.9) | 67 (58.8) |
| Acute myocardial infarction | 15 (62.5) | 6 (27.3) | 10 (43.5) | 10 (43.5) | 13 (59.1) | 39 (43.3) | 54 (47.4) |
| Premature CAD | 6 (25.0) | 5 (22.7) | 6 (26.1) | 4 (17.4) | 6 (27.3) | 21 (23.3) | 27 (23.7) |
| Carotid artery disease, n (%) | 0 | 2 (9.1) | 5 (21.7) | 1 (4.3) | 3 (13.6) | 11 (12.2) | 11 (9.6) |
| Peripheral artery disease, n (%) | 2 (8.3) | 5 (22.7) | 4 (17.4) | 3 (13.0) | 3 (13.6) | 15 (16.7) | 17 (14.9) |
| Stroke, n (%) | 0 | 1 (4.5) | 1 (4.3) | 0 | 1 (4.5) | 3 (3.3) | 3 (2.6) |
| Transient ischaemic attack, n (%) | 2 (8.3) | 1 (4.5) | 0 | 0 | 0 | 1 (1.1) | 3 (2.6) |
| Heart failure, n (%) | 2 (8.3) | 4 (18.2) | 4 (17.4) | 0 | 1 (4.5) | 9 (10.0) | 11 (9.6) |
| Platelet aggregation inhibitors, n (%) | 23 (95.8) | 18 (81.8) | 21 (91.3) | 18 (78.3) | 18 (81.8) | 75 (83.3) | 98 (86.0) |
| Statins, n (%) | 23 (95.8) | 19 (86.4) | 17 (73.9) | 18 (78.3) | 19 (86.4) | 73 (81.1) | 96 (84.2) |
| Ezetimibe, n (%) | 4 (16.7) | 2 (9.1) | 3 (13.0) | 2 (8.7) | 3 (13.6) | 10 (11.1) | 14 (12.3) |
| PCSK9 inhibitor, n (%) | 1 (4.2) | 2 (9.1) | 2 (8.7) | 2 (8.7) | 2 (9.1) | 8 (8.9) | 9 (7.9) |
| Fibrates and/or omega-3 fatty acids, n (%) | 8 (33.3) | 10 (45.5) | 10 (43.5) | 7 (30.4) | 10 (45.5) | 37 (41.1) | 45 (39.5) |
| Anti-diabetic drugs, n (%) | 18 (75.0) | 16 (72.7) | 15 (65.2) | 14 (60.9) | 12 (54.5) | 57 (63.3) | 75 (65.8) |
| Triglyceride, mg/dL | 293.8 ± 86.7 | 281.6 ± 73.4 | 284.8 ± 94.6 | 292.3 ± 89.3 | 268.4 ± 85.1 | 281.9 ± 85.1 | 284.4 ± 85.2 |
| Triglyceride, mg/dL, median (IQR) | 252.0 | 265.3 | 266.0 | 268.5 | 241.8 | 262.0 | 261.5 |
| (222–386) | (230–328) | (211–338) | (218–301) | (222–285) | (222–314) | (222–329) | |
| Triglyceride, mmol/L | 3.32 ± 0.98 | 3.18 ± 0.83 | 3.22 ± 1.07 | 3.30 ± 1.01 | 3.03 ± 0.96 | 3.19 ± 0.96 | 3.21 ± 0.96 |
| Triglyceride, mmol/L, median (IQR) | 2.85 | 3.00 | 3.01 | 3.04 | 2.73 | 2.96 | 2.96 |
| (2.51–4.36) | (2.60–3.72) | (2.38–3.82) | (2.46–3.40) | (2.51–3.22) | (2.51–3.55) | (2.51–3.72) | |
| Apolipoprotein C-III, mg/dL | 16.6 ± 4.5 | 16.0 ± 4.2 | 15.9 ± 4.3 | 16.8 ± 4.2 | 15.7 ± 3.3 | 16.1 ± 4.0 | 16.2 ± 4.1 |
| Apolipoprotein C-III, g/L | 0.17 ± 0.04 | 0.16 ± 0.04 | 0.16 ± 0.04 | 0.17 ± 0.04 | 0.16 ± 0.03 | 0.16 ± 0.04 | 0.16 ± 0.04 |
| Total cholesterol, mg/dL | 144.8 ± 26.0 | 149.2 ± 28.8 | 163.1 ± 35.1 | 160.0 ± 34.6 | 166.8 ± 35.3 | 159.8 ± 33.6 | 156.7 ± 32.7 |
| Total cholesterol, mmol/L | 3.75 ± 0.67 | 3.87 ± 0.75 | 4.22 ± 0.91 | 4.15 ± 0.90 | 4.32 ± 0.91 | 4.14 ± 0.87 | 4.06 ± 0.85 |
| VLDL cholesterol, mg/dL | 53.6 ± 11.7 | 53.9 ± 12.7 | 57.1 ± 24.7 | 53.5 ± 12.1 | 56.6 ± 26.7 | 55.3 ± 19.9 | 54.9 ± 18.4 |
| VLDL cholesterol, mmol/L | 1.39 ± 0.30 | 1.40 ± 0.33 | 1.48 ± 0.64 | 1.39 ± 0.31 | 1.47 ± 0.69 | 1.43 ± 0.52 | 1.42 ± 0.48 |
| Non-HDL cholesterol, mg/dL | 110.3 ± 24.0 | 115.1 ± 28.9 | 129.2 ± 33.3 | 125.3 ± 32.1 | 130.1 ± 31.0 | 125.0 ± 31.4 | 121.9 ± 30.5 |
| Non-HDL cholesterol, mmol/L | 2.85 ± 0.62 | 2.98 ± 0.75 | 3.35 ± 0.86 | 3.24 ± 0.83 | 3.37 ± 0.80 | 3.24 ± 0.81 | 3.16 ± 0.79 |
| LDL cholesterol, mg/dL | 60.2 ± 27.2 | 61.6 ± 18.9 | 70.5 ± 23.2 | 76.2 ± 30.5 | 76.8 ± 20.8 | 71.3 ± 24.2 | 68.9 ± 25.2 |
| LDL cholesterol, mmol/L | 1.56 ± 0.70 | 1.60 ± 0.49 | 1.83 ± 0.60 | 1.97 ± 0.79 | 1.99 ± 0.54 | 1.85 ± 0.63 | 1.79 ± 0.65 |
| Apolipoprotein B, mg/dL | 77.1 ± 19.7 | 79.6 ± 16.6 | 87.9 ± 20.6 | 86.8 ± 19.6 | 88.5 ± 14.3 | 85.7 ± 18.0 | 83.9 ± 18.7 |
| Apolipoprotein B, g/L | 0.77 ± 0.20 | 0.80 ± 0.17 | 0.88 ± 0.21 | 0.87 ± 0.20 | 0.89 ± 0.14 | 0.86 ± 0.18 | 0.84 ± 0.19 |
| HDL cholesterol, mg/dL | 34.6 ± 8.6 | 34.1 ± 9.1 | 33.9 ± 9.5 | 34.8 ± 8.6 | 36.8 ± 10.5 | 34.9 ± 9.4 | 34.8 ± 9.2 |
| HDL cholesterol, mmol/L | 0.90 ± 0.22 | 0.89 ± 0.23 | 0.88 ± 0.25 | 0.90 ± 0.22 | 0.95 ± 0.27 | 0.90 ± 0.24 | 0.90 ± 0.24 |
| Apolipoprotein A1, mg/dL | 131.4 ± 19.3 | 130.2 ± 19.2 | 129.0 ± 16.7 | 132.5 ± 23.0 | 136.3 ± 23.1 | 132.0 ± 20.5 | 131.9 ± 20.2 |
| Apolipoprotein A1, g/L | 1.31 ± 0.19 | 1.30 ± 0.19 | 1.29 ± 0.17 | 1.33 ± 0.23 | 1.36 ± 0.23 | 1.32 ± 0.20 | 1.32 ± 0.20 |
| Lipoprotein(a), nmol/L, median (IQR) | 17.0 | 37.3 | 25.5 | 33.0 | 20.0 | 25.3 | 23.8 |
| (9.0–42.3) | (14.0–170.5) | (13.0–78.0) | (9.0–138.5) | (13.0–70.0) | (12.0–83.0) | (11.0–79.5) | |
| Angiopoietin-like 3, µg/L, median (IQR) | 83.6 | 85.1 | 97.3 | 84.7 | 100.6 | 95.9 | 89.7 |
| (77.4–95.2) | (71.2–109.5) | (81.4–125.4) | (75.4–109.6) | (78.6–119.2) | (75.4–115.5) | (76.4–110.5) | |
| hsCRP, mg/L, median (IQR) | 1.9 | 1.80 | 1.60 | 1.40 | 1.95 | 1.70 | 1.75 |
| (1.25–4.75) | (1.10–3.20) | (1.10–2.50) | (0.90–2.80) | (1.10–6.40) | (1.00–3.20) | (1.10–3.20) | |
| HbA1c (%) | 6.75 ± 1.06 | 6.86 ± 0.94 | 6.80 ± 0.98 | 6.48 ± 0.64 | 6.42 ± 0.90 | 6.64 ± 0.88 | 6.66 ± 0.92 |
Values are given as mean±SD, unless otherwise indicated.
BMI, body mass index; CAD, coronary artery disease; CV, cardiovascular; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; hsCRP, high-sensitivity C-reactive protein; IQR, interquartile range; LDL, low-density lipoprotein; PCSK9, proprotein convertase subtilisin/kexin type 9; SD, standard deviation; VLDL, very-low-density lipoprotein.
At trial entry, 84.2% of patients were being treated with statins and 39.5% with either fibrates or omega-3 fatty acids. The most frequently used anti-diabetic drug was metformin (56.1%). Overall, insulin and insulin analogs were taken by 26.3% of patients, and sodium–glucose co-transporter 2 inhibitors were taken by 23.7% of patients.
At baseline, median [interquartile range (IQR)] fasting triglyceride level was 262 (222–329) mg/dL [2.96 (2.51–3.71) mmol/L], and mean (SD) levels of apoC-III 16.2 (4.1) mg/dL, and VLDL-C 54.9 (18.4) mg/dL [1.42 (0.48) mmol/L] were elevated above normal (approximately twice), while HDL-C levels were slightly decreased [34.8 (9.2) mg/dL] and levels of other lipids/lipoproteins were within normal range.
Primary endpoint-mean percent change in triglyceride level
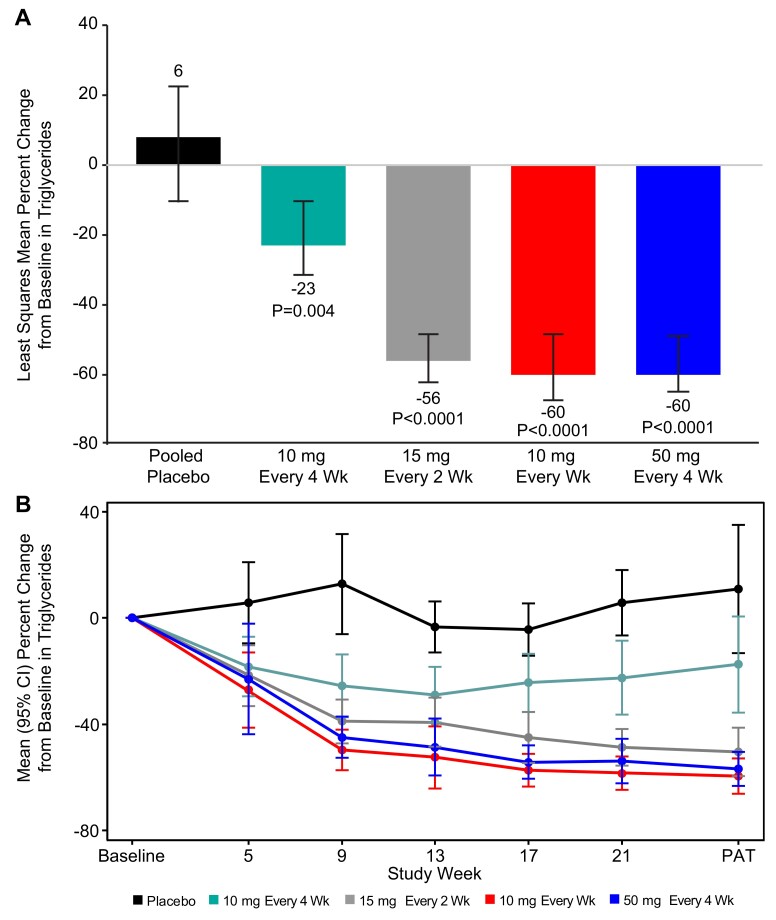
At 6 months of exposure (25 or 27 weeks), statistically significant reductions in fasting triglycerides from baseline were observed in all the olezarsen groups, with least-squares mean (LSM) [95% confidence interval (CI)] changes of –23% (–34 to –10) at a dose of 10 mg every 4 weeks, –56% (–62 to –49) at 15 mg every 2 weeks, –60% (–66 to –54) at 10 mg every week, and –60% (–65 to –53) at 50 mg every 4 weeks, as compared with change of 6% (–9 to 23) for the pooled placebo group (P-value range for the comparison with placebo, 0.0042 to <0.0001) (Figure 1A). The corresponding absolute mean (SD) change from baseline in triglyceride level ranged from –58.8 (102.4) mg/dL [–0.66 (1.16) mmol/L] in the group that received 10 mg every 4 weeks to –184.3 (94.1) mg/dL [–2.08 (1.06) mmol/L] in the group that received 10 mg weekly, while the pooled placebo group showed an absolute increase of 42.8 (206.6) mg/dL [0.48 (2.33) mmol/L]. The triglyceride-lowering effect was observed within the first month of treatment and reached near-maximal effect by Week 17 (in the three higher dose groups) (Figure 1B).
Figure 1.
Effect of olezarsen on fasting triglyceride levels. (A) The least squares mean percent changes in triglycerides from baseline to the primary analysis timepoint. (B) The temporal changes in triglycerides in each of the dose groups. Error bars denote 95% confidence interval. Primary analysis timepoint was Week 27 for weekly dosing, and Week 25 for monthly dosing. The least squares mean of percent change from baseline (95% confidence interval) in each treatment group and the P-value of each olezarsen treatment group vs. the pooled placebo group were estimated using an ANCOVA model with the treatment group as the fixed factor and log-transformed baseline value as the covariate.
Changes in atherogenic lipids and apolipoproteins
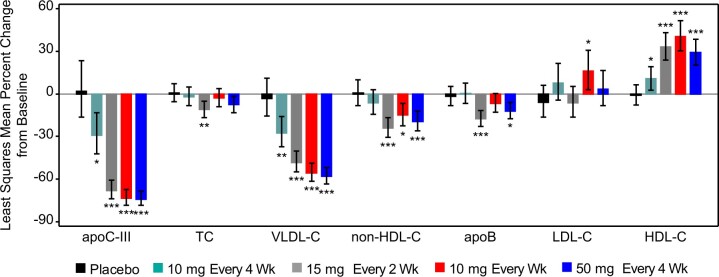
Treatment with olezarsen resulted in significant reductions in levels of atherogenic lipids and apolipoproteins (Table 2 and Figure 2). The LSM (95% CI) changes for the highest monthly dose of 50 mg every 4 weeks were –74% (–80 to –66) for apoC-III (P < 0.0001), –58% (–63 to –52) for VLDL-C (P < 0.0001), –20% (–29 to –9) for non-HDL-C (P = 0.009), and –10% (–19 to –1) for apoB (P = 0.024), compared with placebo at 6 months of exposure. The absolute mean (SD) change from baseline in VLDL-C level ranged from –14.4 (12.3) mg/dL [–0.37 (0.32) mmol/L] in the group that received 10 mg every 4 weeks to –30.4 (13.3) mg/dL [–0.79 (0.34) mmol/L] in the group that received 10 mg weekly, while the placebo group showed absolute increase by 2.4 (20.9) mg/dL [0.06 (0.54) mmol/L]. HDL-C levels increased in all olezarsen treated groups up to 42% (10 mg weekly dose) (P < 0.0001) (Table 2). No dose-dependent effects of olezarsen on LDL-C levels were observed, mean changes in LDL-C varied from 1% reduction at the dose of 15 mg every 2 weeks to 23% increase at the dose of 10 mg weekly (Table 2). The increase in LDL-C in the 10 mg weekly group appeared to be related to changes or discontinuation of in background LDL-C-lowering therapies in some patients.
Table 2.
Percent change from baseline to the primary analysis timepoint in lipids and lipoproteins olezarsen
| Olezarsen | |||||
|---|---|---|---|---|---|
| Placebo | 10 mg | 15 mg | 10 mg | 50 mg | |
| Pooled | Every 4 weeks | Every 2 weeks | Every week | Every 4 weeks | |
| (n = 24) | (n = 22) | (n = 23) | (n = 23) | (n = 22) | |
| Apolipoprotein C-III | |||||
| n | 20 | 18 | 18 | 18 | 21 |
| PAT/baseline, LSM (95% CI) | 1.02 (0.84–1.23) | 0.71 (0.58–0.87) | 0.32 (0.26–0.39) | 0.27 (0.22–0.33) | 0.26 (0.22–0.31) |
| % change from baseline | 2 | –29 | –68 | –73 | –74 |
| PAT/baseline, LSM (95% CI) vs. placebo | 0.70 (0.53–0.92) | 0.32 (0.24–0.42) | 0.26 (0.20–0.35) | 0.26 (0.20–0.34) | |
| % change from baseline vs. placebo | –30 | –68 | –74 | –74 | |
| P-Value | 0.0123 | <0.0001 | <0.0001 | <0.0001 | |
| Triglycerides | |||||
| n | 24 | 22 | 23 | 23 | 22 |
| PAT/baseline, LSM (95% CI) | 1.06 (0.91–1.23) | 0.77 (0.66–0.90) | 0.44 (0.38–0.51) | 0.40 (0.34–0.46) | 0.40 (0.35–0.47) |
| % change from baseline | 6 | 23 | 56 | 60 | 60 |
| PAT/baseline, LSM (95% CI) vs. placebo | 0.73 (0.59–0.90) | 0.42 (0.34–0.52) | 0.37 (0.30, 0.46) | 0.38 (0.31–0.47) | |
| % change from baseline vs. placebo | –27 | –58 | –63 | –62 | |
| P-Value | 0.0042 | <0.0001 | <0.0001 | <0.0001 | |
| VLDL-C | |||||
| n | 20 | 18 | 19 | 18 | 20 |
| PAT/baseline, LSM (95% CI) | 0.97 (0.85–1.11) | 0.73 (0.63–0.84) | 0.52 (0.45–0.60) | 0.44 (0.38–0.51) | 0.42 (0.37–0.48) |
| % change from baseline | –3 | –27 | –48 | –56 | –58 |
| PAT/baseline, LSM (95% CI) vs. placebo | 0.75 (0.61–0.91) | 0.53 (0.44–0.65) | 0.46 (0.38–0.56) | 0.43 (0.36–0.52) | |
| % change from baseline vs. placebo | –25 | –47 | –54 | –57 | |
| P-Value | 0.0049 | <0.0001 | <0.0001 | <0.0001 | |
| Total cholesterol | |||||
| n | 20 | 18 | 19 | 18 | 21 |
| PAT/baseline, LSM (95% CI) | 1.01 (0.94–1.07) | 0.98 (0.92–1.05) | 0.89 (0.83–0.95) | 0.97 (0.91–1.04) | 0.92 (0.87–0.98) |
| % change from baseline | 1 | –2 | –12 | –3 | –8 |
| PAT/baseline, LSM (95% CI) vs. placebo | 0.97 (0.89–1.07) | 0.88 (0.81–0.97) | 0.97 (0.88–1.06) | 0.92 (0.84–1.0) | |
| % change from baseline vs. placebo | –3 | –12 | –3 | –8 | |
| P-Value | 0.5680 | 0.0080 | 0.4476 | 0.0606 | |
| Non-HDL-C | |||||
| n | 20 | 18 | 19 | 18 | 21 |
| PAT/baseline, LSM (95% CI) | 1.01 (0.92–1.10) | 0.94 (0.85–1.03) | 0.76 (0.69–0.83) | 0.85 (0.77–0.93) | 0.81 (0.74–0.88) |
| % change from baseline | 1 | −6 | –24 | –15 | –19 |
| PAT/baseline, LSM (95% CI) vs. placebo | 0.93 (0.82–1.06) | 0.76 (0.66–0.86) | 0.85 (0.74–0.96) | 0.80 (0.71–0.91) | |
| % change from baseline vs. placebo | –7 | –24 | –15 | –20 | |
| P-Value | 0.2826 | <0.0001 | 0.0118 | 0.0009 | |
| Apolipoprotein B | |||||
| n | 20 | 18 | 19 | 18 | 21 |
| PAT/baseline, LSM (95% CI) | 0.98 (0.92–1.05) | 1.00 (0.93–1.08) | 0.83 (0.77–0.88) | 0.93 (0.87–1.00) | 0.88 (0.82–0.94) |
| % change from baseline | –2 | 0 | –17 | –7 | –12 |
| PAT/baseline, LSM (95% CI) vs. placebo | 1.02 (0.93–1.13) | 0.84 (0.76–0.93) | 0.95 (0.86–1.05) | 0.90 (0.81–0.99) | |
| % change from baseline vs. placebo | 2 | –16 | –5 | –10 | |
| P-Value | 0.6801 | 0.0007 | 0.3183 | 0.0240 | |
| LDL-C | |||||
| n | 19 | 18 | 19 | 18 | 18 |
| PAT/baseline, LSM (95% CI) | 0.94 (0.84–1.06) | 1.08 (0.96–1.21) | 0.94 (0.84–1.05) | 1.16 (1.03–1.31) | 1.03 (0.92–1.17) |
| % change from baseline | –6 | 8 | –6 | 16 | 3 |
| PAT/baseline, LSM (95% CI) vs. placebo | 1.14 (0.97–1.35) | 0.99 (0.84–1.17) | 1.23 (1.04–1.45) | 1.10 (0.92–1.30) | |
| % change from baseline vs. placebo | 14 | –1 | 23 | 10 | |
| P-Value | 0.1142 | 0.9486 | 0.0163 | 0.2830 | |
| HDL-C | |||||
| n | 20 | 18 | 19 | 18 | 21 |
| PAT/baseline, LSM (95% CI) | 0.99 (0.92–1.06) | 1.11 (1.03–1.19) | 1.33 (1.24–1.43) | 1.40 (1.30–1.51) | 1.29 (1.20–1.39) |
| % change from baseline | –1 | 11 | 33 | 40 | 29 |
| PAT/baseline, LSM (95% CI) vs. placebo | 1.12 (1.01–1.24) | 1.34 (1.21–1.49) | 1.42 (1.28–1.57) | 1.30 (1.18–1.44) | |
| % change from baseline vs. placebo | 12 | 34 | 42 | 30 | |
| P-Value | 0.0373 | <0.0001 | <0.0001 | <0.0001 | |
| Apolipoprotein A-I | |||||
| n | 20 | 18 | 19 | 18 | 21 |
| PAT/baseline, LSM (95% CI) | 1.00 (0.96–1.04) | 1.05 (1.01–1.10) | 1.14 (1.09–1.19) | 1.18 (1.13–1.23) | 1.14 (1.09–1.18) |
| % change from baseline | 0 | 5 | 14 | 18 | 14 |
| PAT/baseline, LSM (95% CI) vs. placebo | 1.05 (0.99–1.11) | 1.14 (1.08–1.21) | 1.18 (1.11–1.25) | 1.14 (1.07–1.20) | |
| % change from baseline vs. placebo | 5 | 14 | 18 | 14 | |
| P-Value | 0.0936 | <0.0001 | <0.0001 | <0.0001 | |
The least squares means of the PAT to baseline ratio for each treatment group, the ratios of each olezarsen treatment group to the pooled placebo group, the associated 95% CIs, and P-values of each olezarsen treatment group vs. the pooled placebo group were estimated using an ANCOVA model with log-transformed PAT to baseline ratio as the dependent variable, treatment group as the fixed factor, and log-transformed baseline value as the covariate. The estimates obtained from model were converted back to the ratios in original scale. The percent changes from baseline in each treatment group were derived from the PAT to baseline ratios.
CIs, confidence intervals; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LSM, least-squares mean; PAT, primary analysis timepoint; VLDL, very-low-density lipoprotein.
Figure 2.
The least squares mean percent changes in apolipoprotein C-III, total cholesterol, very-low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, apolipoprotein B, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol from baseline to the primary analysis timepoint. Error bars denote 95% confidence interval. primary analysis timepoint was Week 27 for weekly dosing, and Week 25 for monthly dosing.
There were no dose-dependent effects of olezarsen on lipoprotein(a) or ANGPTL3 levels (Supplementary material online, Table S2).
Percent of patients achieving fasting triglyceride level levels < 150 mg/dL (<1.7 mmol/L) or <100 mg/dL (<1.13 mmol/L)
The percent of patients achieving a fasting triglyceride level of <150 mg/dL (<1.7 mmol/L) at 6 months of exposure in olezarsen-treated groups ranged from 14% in the group that received 10 mg every 4 weeks, to 91% in the group that received 50 mg every 4 weeks, while only 4% of patients in the placebo group achieved this threshold (Figure 3A). Fasting triglyceride threshold of <100 mg/dL (<1.13 mmol/L) was achieved by up to 46% of patients treated with olezarsen, and none of placebo patients (Figure 3B).
Figure 3.
Percent of patients achieving fasting triglyceride levels <150 mg/dL (<1.7 mmol/L) (A) and <100 mg/dL (<1.13 mmol/L) (B) at the primary analysis timepoint. The P-values of each olezarsen treatment group vs. the pooled placebo group were estimated using a logistic regression model with the treatment group as the fixed factor and log-transformed baseline value as the covariate.
Safety analyses
Adverse events were similar among groups, with the majority being mild to moderate (Table 3). Serious adverse events occurred in 10% of the patients receiving olezarsen and in 4.2% of those receiving placebo, and none was considered drug-related. One patient with CAD, heart failure, carotid artery disease, aortic stenosis, and Type 2 diabetes mellitus, randomized to olezarsen 15 mg every 2 weeks, died due to cardiac arrest that was reported as not related to the study drug. Treatment discontinuations due to adverse events occurred in 2.2% (2/90) of patients in olezarsen group (cardiac arrest and hypertension) compared with 4.2% (1/24) in the placebo group (eosinophilia and myalgia). The most frequent adverse events in patients treated with olezarsen compared with the placebo group were injection site erythema (15.6% vs. 0%), arthralgia (12.2% vs. 0%), nasopharyngitis (12.2% vs. 8.3%), and upper respiratory tract infection (11.1% vs. 8.3%). The incidence of injection site erythema was the highest in the 10 mg weekly group (21.7%), which also had the highest number of injections. The incidence of other than erythema adverse events at the injection site was low (≤4.4%). Except for one event, each of moderate injection site erythema, pain, and pruritus experienced by one patient from the 10 mg weekly group, all other adverse events at the injection site were reported as mild. The incidence of flu-like reactions was comparable between the pooled olezarsen groups (8.9%) and the pooled placebo groups (8.3%). No patient experienced a confirmed (repeat measure within 1 week) platelet level <100 000/mm3, and no differences compared with placebo were noted in liver chemistry tests, renal function, or clinically significant changes in other laboratory measures including glycaemic parameters, or high-sensitivity C-reactive protein levels, vital signs, or electrocardiographic measures.
Table 3.
Adverse events and laboratory measurements during the treatment perioda
| Pooled | Olezarsen | |||||
|---|---|---|---|---|---|---|
| Placebo | 10 mg | 15 mg | 10 mg | 50 mg | Pooled | |
| (n = 24) | Every 4 weeks | Every 2 weeks | Every weeks | Every 4 weeks | (n = 90) | |
| (n = 22) | (n = 23) | (n = 23) | (n = 22) | |||
| Any adverse event | 20 (83.3) | 17 (77.3) | 20 (87.0) | 22 (95.7) | 21 (95.5) | 80 (88.9) |
| Mild | 10 (41.7) | 8 (36.4) | 9 (39.1) | 15 (65.2) | 11 (50.0) | 43 (47.8) |
| Moderate | 7 (29.2) | 8 (36.4) | 9 (39.1) | 5 (21.7) | 8 (36.4) | 30 (33.3) |
| Severe | 3 (12.5) | 1 (4.5) | 2 (8.7) | 2 (8.7) | 2 (9.1) | 7 (7.8) |
| Serious adverse event | 1 (4.2) | 3 (13.6) | 3 (13.0) | 1 (4.3) | 2 (9.1) | 9 (10.0) |
| Adverse event leading to treatment discontinuation | 1 (4.2) | 0 | 1 (4.3) | 1 (4.3) | 0 | 2 (2.2) |
| Adverse event leading to death | 0 | 0 | 1 (4.3) | 0 | 0 | 1 (1.1) |
| Adverse event at the injection site | 1 (4.2) | 3 (13.6) | 2 (8.7) | 5 (21.7) | 1 (4.5) | 11 (12.2) |
| Flu-like reactionsb | 2 (8.3) | 0 | 3 (13.0) | 4 (17.4) | 1 (4.5) | 8 (8.9) |
| Laboratory measurementsc | ||||||
| Platelet count | ||||||
| <140 000 and ≥100 000/mm3 | 0 | 4 (18.2) | 1 (4.3) | 2 (8.7) | 2 (9.1) | 9 (10.0) |
| <100 000/mm3 | 0 | 0 | 0 | 0 | 0 | 0 |
| ALT level >3× ULN and ≤5× ULN | 0 | 0 | 0 | 0 | 0 | 0 |
| AST level >3× ULN and ≤5× ULN | 0 | 0 | 0 | 0 | 0 | 0 |
| Serum creatinine increase > 0.3 mg/dL from baseline | 2 (8.3) | 2 (9.1) | 1 (4.3) | 0 | 0 | 3 (3.3) |
| eGFR by CKD-EPI >25% decrease from baseline | 4 (16.7) | 4 (18.2) | 4 (17.4) | 3 (13.0) | 4 (18.2) | 15 (16.7) |
| Urine albumin/creatinine ratio >250 mg/g | 0 | 1 (4.5) | 0 | 1 (4.3) | 1 (4.5) | 3 (3.3) |
| Urine protein/creatinine ratio >500 mg/g | 0 | 1 (4.5) | 1 (4.3) | 1 (4.3) | 0 | 3 (3.3) |
For categorical variables, n (%) is presented.
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration formula; eGFR, estimated glomerular filtration rate; ULN, upper limit of normal.
Treatment period was defined as the period from the first dose through one dosing interval after the last dose.
Flu-like reactions are defined as the following adverse events starting on the day of injection or the next day: influenza-like illness, chills, myalgia, arthralgia, pyrexia, feeling hot, or body temperature increased.
Values were confirmed by a second measurement within 7 days. If a second measurement was not available, the result was considered confirmed.
Discussion
This study demonstrates that targeting hepatic APOC3 mRNA with olezarsen in patients at risk of or with established ASCVD results in significant decreases in fasting triglyceride levels by up to 60% compared with placebo. Reflecting the potency of the effectiveness of targeting APOC3 for lowering triglyceride levels, up to 91% of patients achieved a triglyceride level of <150 mg/dL (<1.7 mmol/L). In addition, in the highest dose groups, there were significant and clinically relevant changes in atherogenic lipids and apolipoproteins. olezarsen has the potential to address unmet therapeutic needs in subjects with uncontrolled triglyceride levels and CVD. In the context of the current and other studies targeting APOC3 mRNA, olezarsen may treat the continuum of elevated triglyceride levels, including familial and multifactorial chylomicronemia syndrome, severe hypertriglyceridaemia, and mild hypertriglyceridaemia (Graphical Abstract).
In epidemiology studies, modest elevations of triglyceride levels of 150–500 mg/dL (1.7–5.65 mmol/L) have been shown to be associated with increased risk for ASCVD.2 , 3 , 5 , 22 In subjects with relatively well controlled LDL-C, it is suggested that the remnant cholesterol reflected in the triglyceride measurement may impart a higher risk of cardiovascular events compared with similar levels of cholesterol contained in LDL particles, and this risk is independent of other risk factors.23 , 24 This hypothesis is further supported by observations from the prospective Copenhagen General Population Study showing that a 32 mg/dL reduction in remnant cholesterol, defined mathematically as total cholesterol – (LDL-C + HDL-C), was associated with a 20% relative risk reduction in recurrent major adverse cardiovascular events, compared with a 37 mg/dL reduction in LDL-C and 50 mg/dL in non-HDL-C.22 In the current study, treatment with olezarsen resulted in a mean reduction in VLDL-C levels by 30.4 mg/dL, which would be predicted to result in a similar change in remnant cholesterol and confer a significant reduction in CVD events.
These findings of the current study suggest that inhibiting apoC-III would provide a potent approach in lowering triglyceride levels in a population at high risk for or with established CVD. Besides a robust reduction in apoC-III and triglyceride levels, olezarsen reduced other atherogenic lipoproteins including VLDL-C, non-HDL-C, and apoB and additionally increased HDL-C. The extent of changes in these variables reflected the changes in triglyceride levels in regard to VLDL-C, but with lower reductions in non-HDL-C and apoB. Although the effects of apoB were modest and the change in LDL-C were neutral, the reduction in VLDL-C and non-HDL-C, and by inference remnant cholesterol, suggests a net benefit on atherogenic lipoproteins.
There are several levels of evidence that reducing apoC-III and triglyceride levels support a potential CVD benefit when added to standard of care. First, meta-regression analyses of triglyceride-lowering trials with adjustment for LDL-C demonstrate that to achieve a 20% relative risk reduction, the triglyceride-lowering effect needs to be >100 mg/dL, which has not been achieved to date in an outcomes trial.25 This suggests that higher potency compounds, such as olezarsen, are needed to reduce cardiovascular risk. In the current study, the absolute reduction in triglyceride levels was 184.3 mg/dL (2.08 mmol/L). Second, the magnitude of triglyceride reduction with olezarsen is greater than that observed in studies of heterozygous loss-of-function mutations in APOC3 who had a lifelong ∼40% reduction in triglyceride levels and a corresponding ∼40% reduction in risk of CVD compared with noncarriers.12–14 It is to be acknowledged that these studies also showed an associated modest reduction in LDL-C, therefore the net effect of a more potent pharmacologic intervention on triglyceride lowering but less potent effects on apoB/LDL-C may be difficult to predict.26 Third, there is modest evidence that apoC-III may mediate additional pro-inflammatory responses directly, independent of elevation in triglycerides.9 , 27 , 28
Additional approaches to lowering CVD risk include inhibition of ANGPTL3.29 , 30 A difference between targeting ANGPTL3 vs. apoC-III is that inhibition of ANGPTL3 leads to less potent effects on triglycerides but more potent effects on apoB and LDL-C. Although there are no head-to-head comparison studies, within the context of published data and differences in study baseline characteristics and dose levels, the effect on non-HDL-C may be comparable or perhaps slightly more potent with inhibition of ANGPTL3. Ongoing and future studies with the antisense oligonucleotide vupanorsen (TRANSLATE-TIMI 70, NCT04516291) and small interfering RNAs will provide additional insights into the potential role of inhibiting ANGPTL3 for CVD prevention.
An unresolved issue is whether the ‘triglyceride-lowering hypothesis’ of reducing CVD has been adequately tested. Cardiovascular outcome studies with fibrates as add-on to statin therapy, the most potent approved therapy to lower triglycerides to date, have missed their primary endpoints in the statin era.31 Among omega-3 studies, the REDUCE-IT trial5 showed significant cardiovascular benefit, but the STRENGTH trial did not,6 yet each reduced triglyceride levels similarly by 19%. Thus, the omega-3 studies cannot be used to support the triglyceride-lowering hypothesis and more potent and specific drugs are needed to test this hypothesis.
Olezarsen has a more favourable tolerability and safety profile than volanesorsen and provides an additional option in patients with familial chylomicronemia syndrome (FCS) to reduce the incidence of pancreatitis.10 , 20 FCS is a genetic disorder characterized by homozygous loss of function mutations in LPL and related genes that is associated with extremely elevated triglyceride levels driven primarily by chylomicrons that cannot be cleared due to the lack of adequate LPL activity and the inhibitory effect of apoC-III on clearance mechanisms.32 In the APPROACH study, patients receiving volanesorsen had a 77% decrease in mean triglyceride levels, corresponding to a mean decrease of 1712 mg/dL (19.3 mmol/L) reduction.20 Based on this, volanesorsen is now approved in Europe for FCS. The natural history of FCS can be associated with thrombocytopenia,33 which may be exacerbated by volanesorsen.20 However, major bleeding episodes are not observed with appropriate monitoring of the platelet count. Based on the safety, tolerability, and efficacy findings, olezarsen, which differs from volanesorsen only in its GalNAc moiety allowing a significantly lower dose (cumulative dose 50 mg vs. 1200 mg per month) and less frequent dosing, is currently being studied in the Phase 3 pivotal BALANCE study (NCT04568434—A Study of AKCEA-APOCIII-LRx Administered to Patients With Familial Chylomicronemia Syndrome), a multi-centre, double-blind clinical trial in up to 60 patients randomized to olezarsen 50–80 mg subcutaneously monthly or matching placebo.
Olezarsen may allow treatment optionality of a broader population with multifactorial chylomicronemia and/or triglyceride levels >500–880 mg/dL (>5.65–10.0 mmol/L), which is estimated at 3.4 million subjects in the USA.34 Although several drugs are available for severely elevated triglycerides, such as niacin, fibrates, and omega-3 fatty acids, these are only modestly effective and cannot reduce levels to a normal range. Inhibiting apoC-III has been demonstrated as the most potent approach to reduce triglycerides in such patients, as shown in patients treated with volanesorsen in the COMPASS study.21 Subjects recruited with triglyceride levels >500 mg/dL [baseline ∼1200 mg/dL (13.6 mmol/L)] showed a reduction in mean fasting plasma triglyceride levels by 71%, representing a mean absolute reduction of 869 mg/dL (9.8 mmol/L). In a post-hoc analyses, volanesorsen significantly reduced the incidence of acute pancreatitis, where all cases occurred only in the placebo group. In the current study with lower baseline triglyceride levels, 91% of subjects achieved a triglyceride level of <150 mg/dL (1.7 mmol/L), suggesting that this can be a highly effective method to reduce triglyceride levels below the threshold where acute pancreatitis is most prevalent.
Treatment with olezarsen, a GalNAc-conjugated ASO, was well tolerated with no significant difference in flu-like symptoms compared with placebo and an overall low rate of mild injections site reactions, representing a significant improvement over volanesorsen.19 The drug was also not associated with thrombocytopenia, renal, or hepatic abnormalities, with some patient exposures up to 12 months. To date, thrombocytopenia has not been observed in any studies of GalNAc ASOs.29 , 35 , 36
Limitations
This was a modest-sized, phase 2 b, dose-ranging, safety, and efficacy study and not powered for clinical endpoints. Future studies will need to be performed to address whether targeting apoC-III will reduce cardiovascular events.
In summary, treatment with olezarsen was shown to be safe, well-tolerated, and efficacious with monthly administration, and provides an effective approach to optimally lower triglycerides and TRLs in patients with ASCVD, FCS, and severe hypertriglyceridaemia.
Supplementary material
Supplementary material is available at European Heart Journal online.
Supplementary Material
Acknowledgements
The authors wish to thank Chelsey Jensen and Philip Piscitelli from Akcea Therapeutics for the oversight of the conduct of the study, and Tracy Reigle for generation of the artwork and Shuting Xia for performing the statistical analysis from Ionis Pharmaceuticals.
Funding
Funding was provided by Akcea Therapeutics.
Conflict of interest: C.M.B. has received grant/research support (to his institution) from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostic and has been a consultant for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma Inc, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostic, and Sanofi-Synthelabo. M.D.S. serves on scientific advisory boards: Amgen, Esperion, and Novartis. P.M.M. is a consultant, speaker, or received Research grants from Amgen, Esperion, Kaneka, Amarin, Stage II Innovations/Renew, Novartis, Ionis, FH Foundation, GB Life Sciences, and Aegerion. S.J.B. is a consultant on scientific advisory board or speaker for Altimmune, Akcea, Amgen, AstraZeneca, Boehringer Ingelheim, Axcella, Eli Lilly, Esperion, Madrigal, Novartis, Regeneron, and Sanofi. E.K.-P., E.H., V.J.B., J.K., A.L.F., V.J.A., J.T., R.S.G., L.St.L.O., and S.T. are current or former employees of Ionis/Akcea; J.L.W. is a consultant to Ionis. J.L.W. and S.T. are co-inventors and receive royalties from patents owned by UCSD on oxidation-specific antibodies and of biomarkers related to oxidized lipoproteins and are co-founders and have an equity interest in Oxitope, Inc and its affiliates (‘Oxitope’) as well as in Kleanthi Diagnostics, LLC (‘Kleanthi’). S.T. is a co-founder of Covicept Therapeutics. Although these relationships have been identified for conflict of interest management based on the overall scope of the project and its potential benefit to Oxitope and Kleanthi, the research findings included in this particular publication may not necessarily relate to the interests of Oxitope and Kleanthi. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict of interest policies. D.G. reports grants and personal fees from Akcea Therapeutics and Ionis Pharmaceuticals during the conduct of the study, Arrowhead and Regeneron, and grants from Kowa and Acasti and grants from Uniqure outside the submitted work. The other authors have no disclosures.
Data availability
No data are available.
Appendix
Principal Investigator and Site are: Raimundo Acosta, Bio1 Clinical Research, Miami Beach, FL; Eric St. Amour, Q and T Research Outaouais Inc, Gatineau, QC; Karen Aspry, Miriam Hospital, Providence, RI; Christie Ballantyne, Center for Cardiovascular Disease Prevention, Baylor College of Medicine and DeBakey Heart and Vascular Center, Houston, TX; Cathy Barnes, Suncoast Clinical Research, New Port Richey, FL; Scott Baron, Capitol Interventional Cardiology, Carmichael, CA; Seth Baum, Excel Medical Clinical Trials, LLC, Boca Raton, FL; Harold Bays, L-MARC Research Center, Louisville, KY; Ravi Bhagwat, Cardiovascular Research of Northwest Indiana, LLC, Munster, IN; Samuel Butman, Verde Valley Medical Center, Cottonwood, AZ; Deanna Cheung, Long Beach Center for Clinical Research, Long Beach, CA; James Crenshaw, The Jackson Clinic, PA, Jackson, TN; Anthony DeMaria, University of California San Diego, La Jolla, CA; Isaac Dor, Clinical Investigation Specialists, Inc., Gurnee, IL; Roger Estevez, Clinical Research of South Nevada, Las Vegas, NV; Daniel Gaudet, ECOGENE-21, Chicoutimi, QC; Gary Goldstein, Suncoast Clinical Research, New Port Richey, FL; Thomas Jarrett, Peters Medical Research, High Point, NC; Michael Koren, Jacksonville Center for Clinical Research, Jacksonville, FL; Michael Lillestol, Lillestol Research, LLC, Fargo, ND; Charles Lovell, York Clinical Research, LLC, Norfolk, VA; Steven Lupovitch, Northwest Heart Clinical Research, LLC, Arlington Heights, IL; Ronald Mayfield, Mountain View Clinical Research, Greer, SC; Stephen Miller, Advanced Clinical Research Center, Murray, UT; Rizwana Mohseni, Catalina Research Institute, LLC, Montclair, CA; Patrick Moriarty, University of Kansas Medical Center, Kansas City, KS; Paul Norwood, Valley Research, Fresno, CA; Jason Rasmussen, PMG Research of McFarland Clinic, Ames, IA; Michael Shapiro, Oregon Health and Science University, Portland, OR; Robert Shapiro, PMG Research of McFarland Clinic, Ames, IA; Ehab Sorial NECCR Primacare Research, LLC, Fall River, MA; Jean-Claude, Tardif Montreal Heart Institute, University of Montreal.
Contributor Information
Jean-Claude Tardif, Jean-Claude Tardif MD Research Center, Montreal Heart Institute, 5000 Belanger Street, Montreal, PQ H1T1C8, Canada.
Ewa Karwatowska-Prokopczuk, Akcea Therapeutics, 55 Cambridge Parkway Suite 100 Cambridge, Boston, MA 02142, USA.
Eric St Amour, Eric St-Amour, MD 214 Cite des jeunes Gatineau, QC J8Y 6S8, Canada.
Christie M Ballantyne, Department of Medicine, Baylor College of Medicine, One Baylor Plaza, MS BCM285, Houston, TX 77030, USA.
Michael D Shapiro, Wake Forest University School of Medicine, Section on Cardiovascular Medicine 1, Medical Center Boulevard, Winston-Salem, NC 27157, USA.
Patrick M Moriarty, Division of Clinical Pharmacology, Department of Internal Medicine, University of Kansas Medical Center, 3901 Rainbow Blvd., Kansas City, KS 66160, USA.
Seth J Baum, Clinical Affiliate Professor of Cardiology, Department of Integrated Medical Science, Charles E. Schmidt College of Medicine, Florida Atlantic University, 777 Glades Road, BC-71 Boca Raton, FL 33431, USA.
Eunju Hurh, Akcea Therapeutics, 55 Cambridge Parkway Suite 100 Cambridge, Boston, MA 02142, USA.
Victoria J Bartlett, Akcea Therapeutics, 55 Cambridge Parkway Suite 100 Cambridge, Boston, MA 02142, USA.
Joyce Kingsbury, Akcea Therapeutics, 55 Cambridge Parkway Suite 100 Cambridge, Boston, MA 02142, USA.
Amparo L Figueroa, Akcea Therapeutics, 55 Cambridge Parkway Suite 100 Cambridge, Boston, MA 02142, USA.
Veronica J Alexander, Ionis Pharmaceuticals, Inc., 2855 Gazelle Court, Carlsbad, CA 92010, USA.
Joseph Tami, Ionis Pharmaceuticals, Inc., 2855 Gazelle Court, Carlsbad, CA 92010, USA.
Joseph L Witztum, Division of Endocrinology and Metabolism, University of California, San Diego, 9500 Gilman Drive, BSB1080 La Jolla, CA 92093-0682, USA.
Richard S Geary, Ionis Pharmaceuticals, Inc., 2855 Gazelle Court, Carlsbad, CA 92010, USA.
Louis St L O’Dea, Akcea Therapeutics, 55 Cambridge Parkway Suite 100 Cambridge, Boston, MA 02142, USA.
Sotirios Tsimikas, Ionis Pharmaceuticals, Inc., 2855 Gazelle Court, Carlsbad, CA 92010, USA; Division of Cardiovascular Medicine, University of California, San Diego, 9500 Gilman Drive, BSB1080 La Jolla, CA 92093-0682, USA.
Daniel Gaudet, Department of Medicine, Université de Montréal and Ecogene-21 Clinical Research Centre, Chicoutimi, QC, Canada.
References
- 1. Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet 2014;384:626–635. [DOI] [PubMed] [Google Scholar]
- 2. Schwartz GG, Abt M, Bao W et al. Fasting triglycerides predict recurrent ischemic events in patients with acute coronary syndrome treated with statins. J Am Coll Cardiol 2015;65:2267–2275. [DOI] [PubMed] [Google Scholar]
- 3. Nichols GA, Philip S, Reynolds K, Granowitz CB, Fazio S. Increased cardiovascular risk in hypertriglyceridemic patients with statin-controlled LDL cholesterol. J Clin Endocrinol Metab 2018;103:3019–3027. [DOI] [PubMed] [Google Scholar]
- 4. Laufs U, Parhofer KG, Ginsberg HN, Hegele RA. Clinical review on triglycerides. Eur Heart J 2020;41:99–109c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bhatt DL, Steg PG, Miller M et al. ; REDUCE-IT Investigators . Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 2019;380:11–22. [DOI] [PubMed] [Google Scholar]
- 6. Nicholls SJ, Lincoff AM, Garcia M et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 2020;324:2268–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nordestgaard BG, Langlois MR, Langsted A et al. ; European Atherosclerosis Society (EAS) and the European Federation of Clinical Chemistry and Laboratory Medicine (EFLM) Joint Consensus Initiative . Quantifying atherogenic lipoproteins for lipid-lowering strategies: consensus-based recommendations from EAS and EFLM. Atherosclerosis 2020;294:46–61. [DOI] [PubMed] [Google Scholar]
- 8. Vallejo-Vaz AJ, Fayyad R, Boekholdt SM et al. Triglyceride-rich lipoprotein cholesterol and risk of cardiovascular events among patients receiving statin therapy in the TNT trial. Circulation 2018;138:770–781. [DOI] [PubMed] [Google Scholar]
- 9. Norata GD, Tsimikas S, Pirillo A, Catapano AL. Apolipoprotein C-III: from pathophysiology to pharmacology. Trends Pharmacol Sci 2015;36:675–687. [DOI] [PubMed] [Google Scholar]
- 10. Gaudet D, Brisson D, Tremblay K et al. Targeting APOC3 in the familial chylomicronemia syndrome. N Engl J Med 2014;371:2200–2206. [DOI] [PubMed] [Google Scholar]
- 11. Taskinen MR, Packard CJ, Boren J. Emerging evidence that apoC-III inhibitors provide novel options to reduce the residual CVD. Curr Atheroscler Rep 2019;21:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG, Tybjaerg-Hansen A. Loss-of-function mutations in APOC3 and risk of ischemic vascular disease. N Engl J Med 2014;371:32–41. [DOI] [PubMed] [Google Scholar]
- 13. Crosby J, Peloso GM, Auer PL et al. ; TG and HDL Working Group of the Exome Sequencing Project, National Heart, Lung, and Blood Institute . Loss-of-function mutations in APOC3, triglycerides, and coronary disease. N Engl J Med 2014;371:22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wulff AB, Nordestgaard BG, Tybjærg-Hansen A. APOC3 Loss-of-function mutations, remnant cholesterol, low-density lipoprotein cholesterol, and cardiovascular risk: mediation- and meta-analyses of 137 895 individuals. Arterioscler Thromb Vasc Biol 2018;38:660–668. [DOI] [PubMed] [Google Scholar]
- 15. Wyler von Ballmoos MC, Haring B, Sacks FM. The risk of cardiovascular events with increased apolipoprotein CIII: a systematic review and meta-analysis. J Clin Lipidol 2015;9:498–510. [DOI] [PubMed] [Google Scholar]
- 16. van Capelleveen JC, Bernelot Moens SJ, Yang X et al. Apolipoprotein C-III levels and incident coronary artery disease risk: the EPIC-Norfolk prospective population study. Arterioscler Thromb Vasc Biol 2017;37:1206–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scheffer PG, Teerlink T, Dekker JM et al. Increased plasma apolipoprotein C-III concentration independently predicts cardiovascular mortality: the Hoorn Study. Clin Chem 2008;54:1325–1330. [DOI] [PubMed] [Google Scholar]
- 18. Alexander VJ, Xia S, Hurh E et al. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur Heart J 2019;40:2785–2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gaudet D, Alexander VJ, Baker BF et al. Antisense inhibition of apolipoprotein C-III in patients with hypertriglyceridemia. N Engl J Med 2015;373:438–447. [DOI] [PubMed] [Google Scholar]
- 20. Witztum JL, Gaudet D, Freedman SD et al. Volanesorsen and triglyceride levels in familial chylomicronemia syndrome. N Engl J Med 2019;381:531–542. [DOI] [PubMed] [Google Scholar]
- 21. Gouni-Berthold I, Alexander VJ, Yang Q et al. ; COMPASS Study Group . Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol 2021;9:264–275. [DOI] [PubMed] [Google Scholar]
- 22. Langsted A, Madsen CM, Nordestgaard BG. Contribution of remnant cholesterol to cardiovascular risk. J Intern Med 2020;288:116–127. [DOI] [PubMed] [Google Scholar]
- 23. Castañer O, Pintó X, Subirana I et al. Remnant cholesterol, not LDL cholesterol, is associated with incident cardiovascular disease. J Am Coll Cardiol 2020;76:2712–2724. [DOI] [PubMed] [Google Scholar]
- 24. Balling M, Afzal S, Varbo A et al. VLDL cholesterol accounts for one-half of the risk of myocardial infarction associated with apoB-containing lipoproteins. J Am Coll Cardiol 2020;76:2725–2735. [DOI] [PubMed] [Google Scholar]
- 25. Marston NA, Giugliano RP, Im K et al. Association between triglyceride lowering and reduction of cardiovascular risk across multiple lipid-lowering therapeutic classes: a systematic review and meta-regression analysis of randomized controlled trials. Circulation 2019;140:1308–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cohen JC, Stender S, Hobbs HH. APOC3, coronary disease, and complexities of Mendelian randomization. Cell Metab 2014;20:387–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanter JE, Bornfeldt KE. Apolipoprotein C3 and apolipoprotein B colocalize in proximity to macrophages in atherosclerotic lesions in diabetes. J Lipid Res 2020;62:100010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zewinger S, Reiser J, Jankowski V, Alansary D et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat Immunol 2020;21:30–41. [DOI] [PubMed] [Google Scholar]
- 29. Gaudet D, Karwatowska-Prokopczuk E, Baum SJ et al. ; Vupanorsen Study Investigators . Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur Heart J 2020;41:3936–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graham MJ, Lee RG, Brandt TA et al. Cardiovascular and metabolic effects of ANGPTL3 antisense oligonucleotides. N Engl J Med 2017;377:222–232. [DOI] [PubMed] [Google Scholar]
- 31. Ginsberg HN, Elam MB, Lovato LC et al. ; ACCORD Study Group . Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gordts PL, Nock R, Son NH et al. ApoC-III inhibits clearance of triglyceride-rich lipoproteins through LDL family receptors. J Clin Invest 2016;126:2855–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaudet D, Baass A, Tremblay K et al. Natural history (up to 15 years) of platelet count in 84 patients with familial hyperchylomicronemia due to lipoprotein lipase deficiency. J Clin Lipidol 2017;11:797–798. [Google Scholar]
- 34. Christian JB, Bourgeois N, Snipes R, Lowe KA. Prevalence of severe (500 to 2,000 mg/dl) hypertriglyceridemia in United States adults. Am J Cardiol 2011;107:891–897. [DOI] [PubMed] [Google Scholar]
- 35. Crooke ST, Baker BF, Xia S et al. Integrated assessment of the clinical performance of GalNAc3-conjugated 2′-O-methoxyethyl chimeric antisense oligonucleotides: I. Human volunteer experience. Nucleic Acid Ther 2019;29:16–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsimikas S, Karwatowska-Prokopczuk E, Gouni-Berthold I et al. ; AKCEA-APO(a)-LRx Study Investigators. Lipoprotein(a) reduction in persons with cardiovascular disease. N Engl J Med 2020;382:244–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data are available.