Abstract
AIM
To characterize the neurodevelopmental profile and systemic features of HIVEP2-related disorder.
METHOD
This study used retrospective medical history and standardized assessment data from Simons Searchlight to describe the clinical characteristics of 12 individuals (eight males, four females; age range 3y 3mo–12y 8mo; mean age [SD] 7y 7mo [2y 11mo]) with pathogenic HIVEP2 variants, focusing on their levels of adaptive functioning, autism symptomology, and emotional and behavioral characteristics.
RESULTS
Common features included neonatal complications, hypotonia, developmental delay, intellectual disability, language impairment, gastroesophageal reflux, and strabismus. A minority of individuals had epilepsy, microcephaly, or a movement disorder. Based on the Vineland Adaptive Behavior Scales, Second Edition, affected individuals showed impairments in adaptive behavior (mean composite standard score [SD] 56.4 [10.2]; n=8). The cohort also had significant impairments in social problems, as measured by the Social Responsiveness Scale, Second Edition (mean total score [SD] 76.4 [11.3]; n=10) and clinically significant emotional and behavioral difficulties, as measured by the Child Behavior Checklist for ages 6–18 (mean total T score [SD] 66.9 [8.2]; n=8).
INTERPRETATION
These results show that individuals with HIVEP2-related disorder have impairments in adaptive and social-related behaviors as well as difficulties in emotional and behavioral symptoms.
HIVEP2 (human immunodeficiency virus type I enhancer binding protein 2) encodes a zinc-finger-containing transcription factor that regulates many neurodevelopmental pathways, including NF-κB, somatostatin receptor II, c-Myc, and others that are essential for neuronal development.1–3 HIVEP2 represses transcription of genes in the NF-κB and c-Myc pathways, while activating the effects of somatostatin receptor signaling. In the brain, HIVEP2 is highly expressed in the frontal cortex and hippocampus.1
Hivep2-knockout mice show severe cognitive and social impairments, anxiety-like behaviors, hyperactivity, and memory deficits.3,4 In addition, Hivep2-knockout mice demonstrate chronic inflammation in the brain due to upregulation of NF-κB target genes, suggesting that immune dysregulation may be contributory to the phenotype.3,5
HIVEP2 haploinsufficiency has recently been identified as a rare cause of intellectual disability, with only 14 individuals clinically described in the literature. Among the first reports, clinical features in nine affected individuals included varying degrees of intellectual disability, impaired speech, hypotonia, behavioral symptoms, such as hyperactivity, anxiety, and autism spectrum disorder (ASD), minor structural brain anomalies, and facial dysmorphisms.6,7 An additional study described two affected individuals and broadened the phenotypic spectrum to include hyperphagia and Angelman syndrome-like traits.8 Park et al. characterized two adults with intellectual disability with truncating HIVEP2 variants,9 and Jain et al. described one individual with intellectual disability, hypotonia, and behavioral issues, with a de novo HIVEP2 heterozygous variant.10
Whereas these studies have begun to characterize the clinical characteristics of HIVEP2-related disorder, there are gaps in our knowledge of the prevalence and severity of the developmental, systemic, and neurobehavioral symptoms. Here, we aimed to improve the current understanding of HIVEP2-related disorder through a detailed clinical characterization of 12 individuals using prospective data from the Simons Searchlight project. We particularly focused on standardized measures of adaptive behavior, autism symptomatology, and behavioral and emotional symptoms to assess the neurodevelopmental phenotype of HIVEP2-related disorder.
METHOD
Participants
We obtained data from the Simons Searchlight project, an initiative that recruits and characterizes participants with single gene variants and copy number variants in genes associated with ASD and intellectual disability (https://www.sfari.org/resource/simons-searchlight). We accessed data for this manuscript on 18th October 2020. In this study, we included probands with pathogenic or likely pathogenic variants in HIVEP2.
Instruments
Demographic information and medical history from the family was collected through an online parent-reported questionnaire (Tables 1 and 2). The patients’ age at which this information was obtained is listed in Table 1. All data collection in Simons Searchlight is conducted remotely and longitudinally. We used the most recent data with the oldest age available for 10 individuals who completed a comprehensive, structured medical history interview with one of two licensed genetic counselors. The medical history interview covers all major medical conditions and the developmental and psychiatric diagnostic history. Diagnoses such as ASD were documented through parental report of prior professional evaluations, including by pediatricians, developmental pediatricians, neurologists, psychologists, and clinical geneticists. Information regarding original ASD assessment methods was not available. The remaining two participants did not complete the medical history interview but completed the online seizure history survey, which was used to supplement medical information.
Table 1:
Demographic and genetic characteristics of participants in the cohort
| P 1 | P 2 | P 3 | P 4 | P 5 | P 6 | P 7 | P 8 | P 9 | P 10 | P 11 | P 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | Male | Female | Female | Female | Male | Female | Male | Male | Male | Male | Male | Male |
| Ethnic group | White | White | White | >1 (Asian, White) | Asian | White | White | White | White | White | White | ND |
| Latest age of participant at which medical history was ascertained | 6y 2mo | 10y 3mo | 6y 9mo | 11y | 6y 8mo | 4y 3mo | 5y 6mo | 11y 2mo | 6y 10mo (seizure evaluation only) | 6y 11mo | 3y 3mo | 12y 8mo (seizure evaluation only) |
| Variant | c.1189G>T p.Asp397Tyr |
c.2827C>T p.Arg943* |
c.6964C>T p.Gln2322* |
c.2905C>T p.Gln969* |
c.5686C>T p.Gln1896* |
c.5900delC p.Ser1967Cysfs |
c.2827C>T p.Arg943* |
c.5935C>T p.Arg1979* |
c.3742C>T p.Gln1248* |
c.6667C>T p.Arg2223* |
c.5150dupA p.Leu1718Alafs |
c.5935C>T p.Arg1979* |
| Type of variant | Missense | Nonsense | Nonsense | Nonsense | Nonsense | Frameshift | Nonsense | Nonsense | Nonsense | Nonsense | Frameshift | Nonsense |
| Inheritance | De novo | De novo | De novo | De novo | De novo | Inherited | De novo | Unknown | De novo | De novo | De novo | Unknown |
| ACMG classification | Likely pathogenic | Pathogenic | Likely pathogenic | Pathogenic | Pathogenic | Pathogenic | Pathogenic | Likely pathogenic | Pathogenic | Pathogenic | Pathogenic | Pathogenic |
P, participant; ND, data not available; ACMG, American College of Medical Genetics.
Table 2:
Clinical characteristics of participants in the cohort
| Overall% | P 1 | P2 | P 3 | P 4 | P 5 | P 6 | P 7 | P 8b | P 9 | P 10 | P 11 | P 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnancy and neonatal | |||||||||||||
|
| |||||||||||||
| Complications during pregnancy | 40 | Yes (polyhydramnios) | Yes (bleeding) | No | Yes (gestational diabetes) | Yes (gestational diabetes, bleeding) | No | No | No | NA | No | No | NA |
| Gestational age (wks) | 39 | 37 | 40 | 37 | 40 | 39 | 40 | 35 | NA | 39 | 39 | NA | |
| Birthweight, kg (z-score)a | 4.1 (+1.4) | 3.1 (−0.3) | 3.7 (+1.0) | 2.6 (−1.5) | 3.3 (−0.1) | 3.5 (+0.6) | 3.7 (+0.7) | 2.3 (−0.5) | NA | 2.8 (−1.2) | 4.2 (+1.6) | NA | |
| Neonatal complications | 90 | Yes (hypotonia, feeding difficulty, lethargy) | Yes (hypotonia, hyperbilirubinemia) | Yes (hypoglycemia) | Yes (temperature regulation, hyperbilirubinemia) | Yes (feeding difficulty, irritability, respiratory distress, hyperbilirubinemia) | Yes (meconium) | Yes (hypotonia) | Yes (feeding difficulty, respiratory distress, hyperbilirubinemia) | NA | No | Yes (poor suck, feeding difficulty, irritability) | NA |
|
| |||||||||||||
| Neurodevelopmental and psychiatric | |||||||||||||
|
| |||||||||||||
| Autism spectrum disorder | 50 | Yes | Yes | No | Yes | Yes | No | No | No | NA | No | Yes | NA |
| Intellectual disability/developmental delay | 100 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | Yes | NA |
| Language delay or impairment | 60 | Yes | No | No | No | No | Yes | Yes | Yes | NA | Yes | Yes | NA |
| History of seizures | 25 | Yes | No | No | Yes | Yes | No | No | No | No | No | No | No |
| Current diagnosis of epilepsy | 17 | Yes | No | No | No | Yes | No | No | No | No | No | No | No |
| Macrocephaly | 10 | No | No | No | No | No | No | No | No | NA | No | Yes | NA |
| Microcephaly | 30 | Yes | No | No | No | Yes | No | No | Yes | NA | No | No | NA |
| Cerebral palsy | 30 | No | Yes | Yes | No | Yes | No | No | No | NA | No | No | NA |
| Hypotonia | 90 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NA | Yes | No | NA |
| Movement disorder | 40 | Yes | Yes | No | No | Yes | Yes | No | No | NA | No | No | NA |
| ADHD | 10 | Yes | No | No | No | No | No | No | No | NA | No | No | NA |
| Anxiety | 10 | No | No | No | No | No | No | No | Yes | NA | No | No | NA |
|
| |||||||||||||
| Systemic | |||||||||||||
|
| |||||||||||||
| Vision | |||||||||||||
|
| |||||||||||||
| Hyperopia | 40 | Yes | No | Yes | No | Yes | No | No | No | NA | Yes | No | NA |
| Myopia | 10 | No | No | No | No | No | No | No | No | NA | Yes | No | NA |
| Strabismus | 50 | No | Yes | Yes | No | Yes | No | Yes | Yes | NA | No | No | NA |
| Astigmatism | 10 | No | No | No | No | Yes | No | No | No | NA | No | No | NA |
| Respiratory: asthma | 20 | Yes | No | No | Yes | No | No | No | No | NA | No | No | NA |
| Gastrointestinal | |||||||||||||
| Reflux | 50 | No | Yes | Yes | No | Yes | No | Yes | No | NA | Yes | No | NA |
| Constipation | 30 | No | Yes | No | No | No | No | No | No | NA | Yes | Yes | NA |
| Failure to thrive | 20 | Yes | No | No | No | Yes | No | No | No | NA | No | No | NA |
| Genitourinary: vesicoureteral reflux | 10 | No | No | Yes | No | No | No | No | No | NA | No | No | NA |
| Musculoskeletal: hip dysplasia | 10 | No | Yes | No | No | No | No | No | No | NA | No | No | NA |
| Dermatological: eczema | 20 | No | No | No | Yes | Yes | No | No | No | NA | No | No | NA |
| Immunodeficiency | 11 | No | Yes | No | No | No | No | No | No | NA | NA | No | NA |
For birthweight, z-scores were calculated using the World Health Organization infant weight for age charts for infants born at 37 weeks’ gestation and older.18
For Participant 8, the z-score was calculated using the Fenton 2013 growth chart for preterm infants.19 NA, data not available; ADHD, attention-deficit/hyperactivity disorder.
We further used the Vineland Adaptive Behaviors Scales, Second Edition (VABS-II) interview, the Social Responsiveness Scale, Second Edition (SRS-2), the Social Communication Questionnaire - Lifetime (SCQ), and the Child Behavior Checklist for ages 6–18 (CBCL/6–18). All these instruments were administered as structured interviews by a genetic counselor via Simons Searchlight. For the VABS-II, SRS-2, SCQ, and CBCL/6–18, if there was more than one time point for each individual we used the most recently obtained data.
The VABS-II assesses adaptive abilities and consists of an overall composite score and individual subdomain scores for motor, communication, daily living, and socialization skills.11 The composite score and subdomain scores are standardized to a mean of 100 with a standard deviation (SD) of 15, with lower scores indicating greater severity of impairment. As in prior studies,12 we used a score of 70 to 84 (−1 to −2 SD) to indicate borderline impairment, 55 to 69 (−2 to −3 SD) to indicate mild impairment, 40 to 54 (−3 to −4 SD) to indicate moderate impairment, 25 to 39 (−4 to −5 SD) to indicate severe impairment, and 24 or less to indicate profound impairment. VABS-II data were available for eight out of 12 individuals and were obtained at an average age of 7 years 6 months.
The SRS-2 measures impairments in social and other behavior and is used to screen for the risk of ASD.13 It reports total scores as well as five subscale scores: social awareness, social cognition, social communication, social motivation, and mannerisms. Raw scores are converted to normed T scores, where a T score of 59 or less is considered normal, scores of 60 to 65 indicate mild impairments in social behavior, scores of 66 to 75 indicate moderate impairments in social behavior, and scores of 76 or higher are considered severe. SRS-2 data were available for 10 out of 12 individuals and were obtained at an average age of 8 years 10 months.
The SCQ is a screening instrument consisting of 40 ‘yes/no’ questions, originally designed to evaluate communication and social skills in children over 4 years of age suspected to have ASD.14 It is also used in research studies to compare symptomology in children with ASD and other clinical groups and to track changes over time. The Lifetime form focuses on symptomology throughout the child’s entire development, and scores above the cut-off of 15 suggest a stronger possibility of ASD.15 SCQ data were available for 10 of the 12 individuals and were obtained at an average age of 7 years 3 months.
The CBCL/6–18 is a caregiver questionnaire using a Likert scale that seeks to identify behavioral and emotional problems in children and adolescents using eight syndrome subscales and two broadband scales.16 The broadband internalizing domain includes three syndrome scales: anxious/depressed, withdrawn/depressed, and somatic complaints. The broadband externalizing domain combines two syndrome scales: rule-breaking behavior and aggressive behavior. In addition, there are three other syndrome scales: social problems, thought problems, and attention problems. A total problems scale assesses the individual’s overall level of impairment and is derived from the scores of all eight syndrome scales. Raw scores can be converted into normed T scores (mean of 50 [SD 10]). T scores of 64 or greater are considered to be clinically significant on the two broadband scales and the total problems scale, whereas T scores of 70 or greater are considered clinically significant in the individual syndrome scales. Scores between 60 and 63 and between 65 and 69 are considered borderline for the broadband and total problems scales and the individual syndrome scales respectively.16,17 CBCL/6–18 data were available for eight of the 12 participants and were obtained at an average age of 9 years 4 months.
For ethics approval, all Searchlight participants provided informed consent and the study was reviewed by the Institutional Review Boards at Geisinger and Columbia. All data were deidentified by Simons Searchlight and access to this data was approved by Boston Children’s Hospital Institutional Review Board.
RESULTS
Table 1 shows the demographic and genetic information of the 12 participants in the cohort. Four of the 12 participants were female and the age of individuals at the medical history and seizure history evaluations ranged from 3 years 3 months to 12 years 8 months (Table 1). Nine participants had a nonsense variant, one had a missense variant, and two had a frameshift variant (Fig. 1). The variants clustered in exons 5, 9, and 10. In cases for whom the inheritance was known, all but one had a de novo variant. Participant 11 showed somatic mosaicism, with 23% of sequencing reads showing the HIVEP2 variant. Participant 6 inherited an HIVEP2 variant from an unaffected parent, thought to be in the setting of germline mosaicism in that parent. We excluded the asymptomatic parent from this study.
Figure 1:
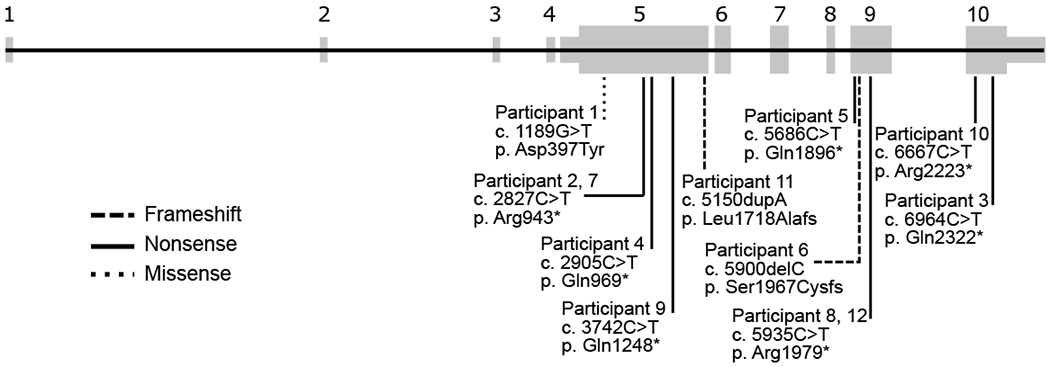
Schematic of variants in HIVEP2 in the Simons Searchlight project.
All participants except one were born at term (Table 2). Nine out of 10 participants had neonatal complications that included hyperbilirubinemia, hypoglycemia, temperature dysregulation, meconium, respiratory distress, hypotonia, feeding difficulty, poor suck, lethargy, and irritability.
Among neurological features, three of 12 participants had a history of at least one seizure, and two had a current diagnosis of epilepsy (Table 2). Participant 1 was placed on lamotrigine, whereas participant 5 was taking both levetiracetam and valproic acid. One of 10 participants had macrocephaly, while three of 10 had microcephaly. Three of 10 participants had a diagnosis of cerebral palsy. Nine of 10 individuals had hypotonia; four of 10 had a movement disorder.
In terms of developmental disorders, five of 10 participants had a diagnosis of ASD (Table 2). All individuals were reported to have developmental delay and/or intellectual disability, although standardized IQ scores were not available for analysis. Six of 10 participants had a formal diagnosis of language delay or impairment. One of 10 had a diagnosis of attention-deficit/hyperactivity disorder (ADHD) and one other participant had anxiety. Despite the lack of a formal diagnosis of anxiety and ADHD respectively, three individuals were placed on a selective serotonin reuptake inhibitor, and two participants were placed on a stimulant medication. Other common medical features were gastroesophageal reflux (5/10), strabismus (5/10), hyperopia (4/10), constipation (3/10), failure to thrive (2/10), eczema (2/10), and asthma (2/10) (Table 2).
We examined patterns of adaptive behavior, social challenges, and behavioral and emotional problems in our cohort, using the VABS-II, SRS-2, and CBCL/6–18 respectively. Participants showed impairments in all domains of adaptive behavior measured by the VABS-II (Fig. 2a). The mean VABS-II composite standard score was 56.4 (SD 10.2, n=8), indicating, on average, a mild impairment in adaptive abilities. In addition, scores in the mildly impaired range were observed, on average, across participants in each VABS-II subdomain (motor, communication, daily living, and socialization skills). Scores for daily living skills showed the greatest range across individuals, with one individual showing borderline impairment, three individuals showing mild impairment, three individuals showing moderate impairment, and one individual showing severe impairment.
Figure 2.
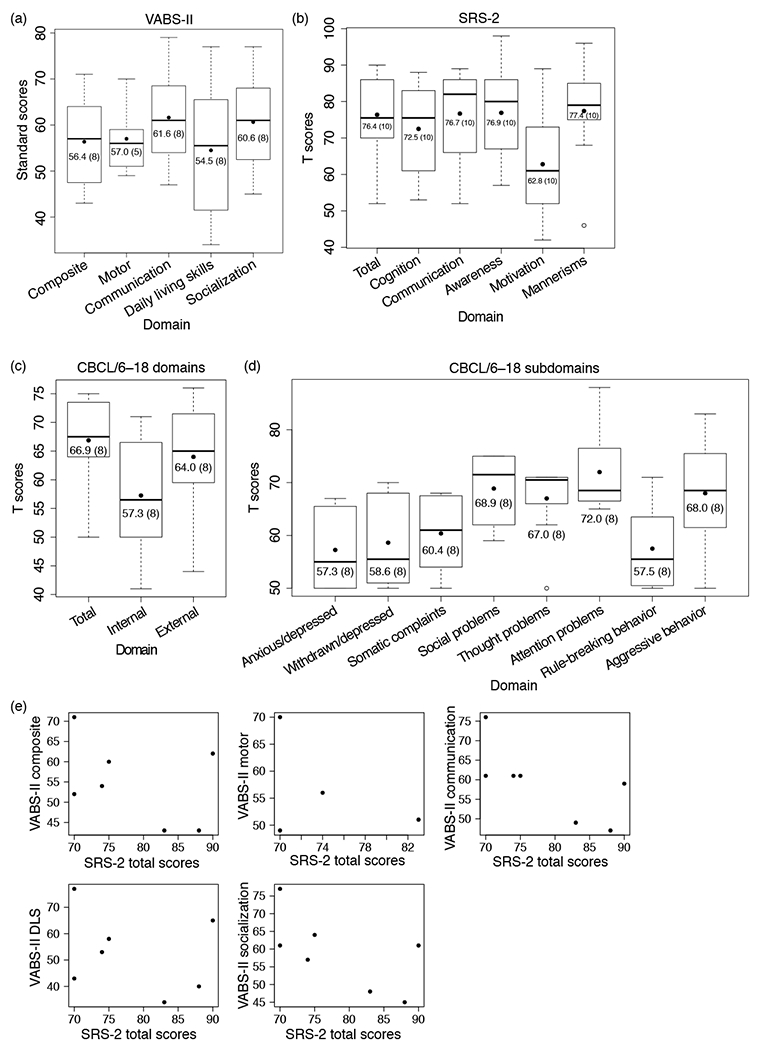
Box-and-whisker plots of (a) the Vineland Adaptive Behavior Scales, Second Edition (VABS-II) standard scores (overall composite and individual subdomains), (b) the Social Responsiveness Scales, Second Edition (SRS-2) T scores (total and individual subscales), (c) the Child Behavioral Checklist for ages 6–18 (CBCL/6–18) T scores for total score, internal broadband scale, and external broadband scale, and (d) CBCL/6–18 T scores for individual syndrome scales in the cohort. The black dot indicates the mean score in each category. VABS-II scores are standardized to a mean of 100 (SD 15). Lower scores indicate greater impairment. For the SRS-2, scores ≤59 are considered normal and higher scores indicate greater impairment. CBCL/6–18 scores are scaled to a mean of 50 (SD 10). Higher scores indicate greater impairment. The mean value for each score is listed, followed by the number of individuals included in the analysis in parentheses. (e) Relationship between autism spectrum disorder symptom severity (SRS-2 total T score) and adaptive functioning (VABS-II composite and subdomain standard scores). Seven individuals had available SRS-2 scores together with VABS-II composite, communication, daily living skills, and socialization scores; only four individuals had available SRS-2 scores together with VABS-II motor scores. DLS, daily living skills.
The cohort also showed significant impairments in social and other behaviors, as shown by the mean cohort total SRS-2 score of 76.4 (SD 11.3, n=10), which would be classified in the severe range (Fig. 2b).13 Both individuals with and without an ASD diagnosis had elevated SRS-2 total scores (with ASD, mean 79.0 [SD 8.0], n=4; without ASD, mean 71.5 [SD 15.6], n=4; p=0.44; Welch two-sample t-test). Similarly, both individuals with and without an ASD diagnosis had elevated SCQ summary scores (with ASD, mean 17.6 [SD 5.5], n=5; without ASD, mean 17.3 [SD 6.7], n=3; p=0.96; Welch two-sample t-test).
The participants showed clinically significant difficulties in emotional and behavioral symptoms, with an average CBCL/6–18 total T score across participants of 66.9 (SD 8.2, n=8) (Fig. 2c). The internalizing problems broadband T score (mean 57.3 [SD 10.7] n=8) fell within the normal reference range, whereas the externalizing problems broadband T score was considered clinically significantly elevated (mean 64.0 [SD 10.2] n=8). The T score for the subdomain of attention problems (mean 72.0 [SD 8.6], n=8) was considered clinically significantly elevated (Fig. 2d). T scores pertaining to the CBCL/6–18 subdomains of social problems (mean 68.9 [SD 6.9] n=8), thought problems (mean 67.0 [SD 7.5] n=8), and aggressive behaviors (mean 68.0 [SD 10.6] n=8) were borderline elevated, whereas the scores of the remaining subdomains were not clinically significant.
We then asked whether there was a correlation between ASD symptom severity and adaptive functioning. We used the SRS-2 total T score as a proxy for ASD symptom severity and VABS-II scores (total and subscale standard scores) to measure adaptive functioning. We observed negative trends between the SRS-2 total T score and composite and subdomain VABS-II standard scores (Fig. 2e), but correlations between the variables were not statistically significant due to the small sample sizes. This analysis suggests that increased ASD symptom severity is associated with lower adaptive functioning in individuals with HIVEP2 mutations.
DISCUSSION
Consistent with prior studies, we found that participants with HIVEP2-related disorder in the Simons Searchlight cohort have a range of neurodevelopmental phenotypes, including developmental delay/intellectual disability, ASD, and hypotonia. Hypotonia may be a contributing factor for the motor deficits as well as gastroesophageal reflux and feeding difficulties. Less commonly, affected individuals have epilepsy, movement disorder, cerebral palsy, ADHD, and anxiety. Three of 10 of our cohort had microcephaly, which has been reported in prior studies.7 One individual was noted to have macrocephaly; more studies are needed to determine if macrocephaly may also be a feature of HIVEP2-related disorder.
A strength of our study is the use of standardized assessment tools to characterize adaptive functioning, social impairments, and behavioral challenges. We found that, as a group, affected participants showed substantial impairments in all aspects of adaptive behavior, as measured by the VABS-II, impairments in social and other behaviors, as measured by the SRS-2, and clinically significant difficulties in emotional and behavioral symptoms, as measured by the CBCL/6–18. Furthermore, we observed a trend that increased ASD symptom severity was associated with lower adaptive functioning in individuals with HIVEP2-related disorder. The data in our study regarding cognitive development will help in counseling families with a new diagnosis of HIVEP2-related disorder in their child about expectations and management.
The participants showed elevated scores in the SRS-2 and SCQ scales irrespective of ASD diagnosis, suggesting the presence of behaviors in affected individuals that may be consistent with those seen in ASD, even in those without a formal ASD diagnosis. Another possibility is that ASD may be underdiagnosed in those with HIVEP2-related disorder due to the difficulty of diagnosing ASD in individuals with concurrent intellectual disability.20
There are several limitations to our study. One weakness is the lack of standardized IQ scores for analysis and the reliance on parental report of diagnoses and developmental delays. However, adaptive functioning scores may be a proxy for IQ, given the correlation between these two measures seen in prior ASD research.21 Another limitation is the small sample size for this ultra-rare disorder, which precludes the ability to draw statistical comparisons. In addition, we could not confidently remove or identify participants overlapping with prior reports. Nevertheless, this study adds to the literature by being the first to characterize HIVEP2-related disorder through standardized developmental and behavioral tools. We advise caution in interpreting the results of the CBCL/6–18 (a tool originally designed for typically developing children) in individuals with intellectual disability, such as the HIVEP2-related disorder population. A study in a trisomy 21 population identified concerns with the CBLC internal consistency and interrater reliability in the internalizing subscales.22 However, a study on 152 individuals with a variety of neurogenetic disorders suggested that the CBCL/6–18 is a psychometrically sound narrowband and broadband measure of difficult behaviors.23
In summary, this study confirms prior neurological phenotypes and identifies new and more precise neurobehavioral characteristics of HIVEP2-related disorder, which will help provide guidance for clinicians and families of affected individuals. Further work should include additional clinical phenotyping and mechanistic studies on how pathogenic HIVEP2 variants affect the developing brain.
What this paper adds.
Common features of HIVEP2-related disorder include developmental delay, hypotonia, and autism symptomology.
Individuals show impairments in adaptive behavior measured by the Vineland Adaptive Behavior Scales, Second Edition.
Individuals show impairments in social-related behaviors measured by the Social Responsiveness Scale, Second Edition.
Individuals show difficulties in emotional and behavioral symptoms as measured by the Child Behavior Checklist.
Acknowledgements
We are grateful to all the families participating in Simons Searchlight as well as data from Simons Searchlight. Support to WKC is provided by a grant from Simons Foundation Autism Research Initiative. Support to SS is provided by National Institute of Neurological Disorders and Stroke 1K23NS119666. The authors have stated that they had no interests that might be perceived as posing a conflict or bias.
ABBREVIATIONS
- ASD
Autism spectrum disorder
- CBCL/6–18
Child Behavior Checklist for ages 6–18
- VABS-II
Vineland Adaptive Behaviors Scales, Second Edition
- SRS-2
Social Responsiveness Scale, Second Edition
- SCQ
Social Communication Questionnaire - Lifetime
Data availability statement
Approved researchers can obtain the data set described in this study by applying at https://base.sfari.org.
References
- 1.Dörflinger U, Pscherer A, Moser M, Rümmele P, Schüle R, Buettner R. Activation of somatostatin receptor II expression by transcription factors MIBP1 and SEF-2 in the murine brain. Mol Cell Biol 1999; 19: 3736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fukuda S, Yamasaki Y, Iwaki T, et al. Characterization of the biological functions of a transcription factor, c-myc intron binding protein 1 (MIBP1). J Biochem 2002; 131: 349–57. [DOI] [PubMed] [Google Scholar]
- 3.Takao K, Kobayashi K, Hagihara H, et al. Deficiency of schnurri-2, an MHC enhancer binding protein, induces mild chronic inflammation in the brain and confers molecular, neuronal, and behavioral phenotypes related to schizophrenia. Neuropsychopharmacology 2013; 38: 1409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takagi T, Jin W, Taya K, Watanabe G, Mori K, Ishii S. Schnurri-2 mutant mice are hypersensitive to stress and hyperactive. Brain Res 2006; 1108: 88–97. [DOI] [PubMed] [Google Scholar]
- 5.Choi J-K, Zhu A, Jenkins BG, et al. Combined behavioral studies and in vivo imaging of inflammatory response and expression of mGlu5 receptors in schnurri-2 knockout mice. Neurosci Lett 2015; 609: 159–64. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava S, Engels H, Schanze I, et al. Loss-of-function variants in HIVEP2 are a cause of intellectual disability. Eur J Hum Genet 2016; 24: 556–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinfeld H, Cho MT, Retterer K, et al. Mutations in HIVEP2 are associated with developmental delay, intellectual disability, and dysmorphic features. Neurogenetics 2016; 17: 159–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldsmith H, Wells A, Sá MJN, et al. Expanding the phenotype of intellectual disability caused by HIVEP2 variants. Am J Med Genet A 2019; 179: 1872–7. [DOI] [PubMed] [Google Scholar]
- 9.Park J, Colombo R, Schäferhoff K, et al. Novel HIVEP2 variants in patients with intellectual disability. Mol Syndromol 2019; 10: 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Atwal PS. Novel HIVEP2 Variant p. Q1248* is Associated with Developmental Delay: A Case Report. J Pediatr Genet 2019; 8: 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sparrow SS, Cicchetti D, Balla DA. Vineland adaptive behavior scales-2nd edition manual. Minneapolis, MN: NCS Pearson Inc., 2005. [Google Scholar]
- 12.Farmer C, Adedipe D, Bal VH, Chlebowski C, Thurm A. Concordance of the Vineland Adaptive Behavior Scales, second and third editions. J Intellect Disabil Res 2020; 64: 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantino J, Gruber C. The Social Responsiveness Scale Manual, Second Edition (SRS-2). Los Angeles, CA: Western Psychological Services, 2012. [Google Scholar]
- 14.Rutter M, Bailey A, Lord C. The social communication questionnaire: Manual. Los Angeles, CA: Western Psychological Services, 2003. [Google Scholar]
- 15.Berument SK, Rutter M, Lord C, Pickles A, Bailey A. Autism screening questionnaire: Diagnostic validity. Br J Psychiatry 1999; 175: 444–51. [DOI] [PubMed] [Google Scholar]
- 16.Achenbach TM, Rescorla L. Manual for the ASEBA school-age forms & profiles: An integrated system of multi-informant assessment. Burlington, VT: ASEBA, 2001. [Google Scholar]
- 17.Pandolfi V, Magyar CI, Dill CA. An initial psychometric evaluation of the CBCL 6-18 in a sample of youth with autism spectrum disorders. Res Autism Spectr Disord 2012; 6: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. The WHO Child Growth Standards. Available at: www.who.int/childgrowth/standards/en/ (accessed 1 June 2021).
- 19.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013; 13: 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thurm A, Farmer C, Salzman E, Lord C, Bishop S. State of the field: Differentiating intellectual disability from autism spectrum disorder. Front Psychiatry 2019; 10: 526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathak M, Bennett A, Shui AM. Correlates of adaptive behavior profiles in a large cohort of children with autism: The autism speaks Autism Treatment Network registry data. Autism 2019; 23: 87–99. [DOI] [PubMed] [Google Scholar]
- 22.Esbensen AJ, Hoffman EK, Shaffer R, Chen E, Patel L, Jacola L. Reliability of parent report measures of behaviour in children with Down syndrome. J Intellect Disabil Res 2018; 62: 785–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neo WS, Suzuki T, Kelleher BL. Structural validity of the Child Behavior Checklist (CBCL) for preschoolers with neurogenetic syndromes. Res Dev Disabil 2021; 109: 103834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Approved researchers can obtain the data set described in this study by applying at https://base.sfari.org.


