Figure 1: Regulatory mechanisms controlling NA-sensing TLR activation.
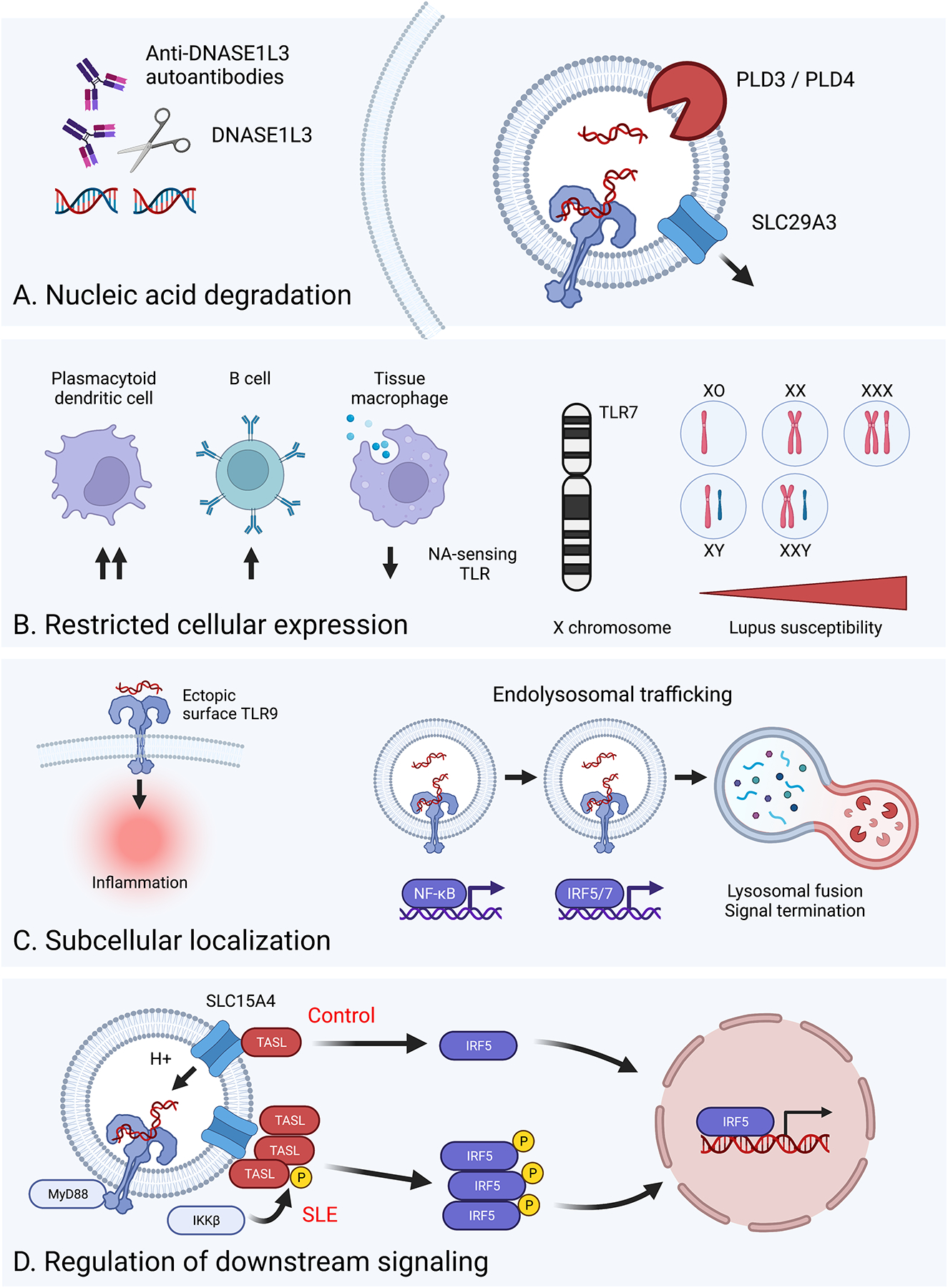
Schematic detailing the various strategies employed by the immune system to limit aberrant activation of NA-sensing TLRs. A. Nucleic acid degradation: Expression of specific nucleases, such as DNASE1L3, PLD3, and PLD4, or the lysosomal transporter SLC29A3, degrade or remove self-NA prior to recognition by NA-sensing TLRs. This regulatory mechanism can be subverted by either genetic defects in specific genes or via the development of anti-DNASE1L3 autoantibodies. B. Restricted cellular expression: The expression of NA-sensing TLRs is limited to certain immune lineages, such as pDCs and B cells, while specifically reduced on tissue macrophages designed for apoptotic cell clearance (left). Aberrant activation of NA-sensing TLRs is further controlled via regulated mRNA expression, as evidenced by X chromosome gene dosage driving TLR7-dependent lupus pathogenesis (right). C. Subcellular localization: NA-sensing TLRs traffic to endosomal compartments to prevent pathogenic engagement by circulating NA (left). Following endosomal TLR ligation, induction of endolysosomal trafficking serves to both control the amplitude of and terminate TLR signaling (right). D. Regulation of downstream signaling: Amongst many genetic variants impacting lupus risk, CXorf21 polymorphisms increase TASL levels resulting in increased TLR-dependent nuclear translocation of IRF5.
