Figure 3: Impact of non-canonical autophagy gene variants on endosomal TLR signals.
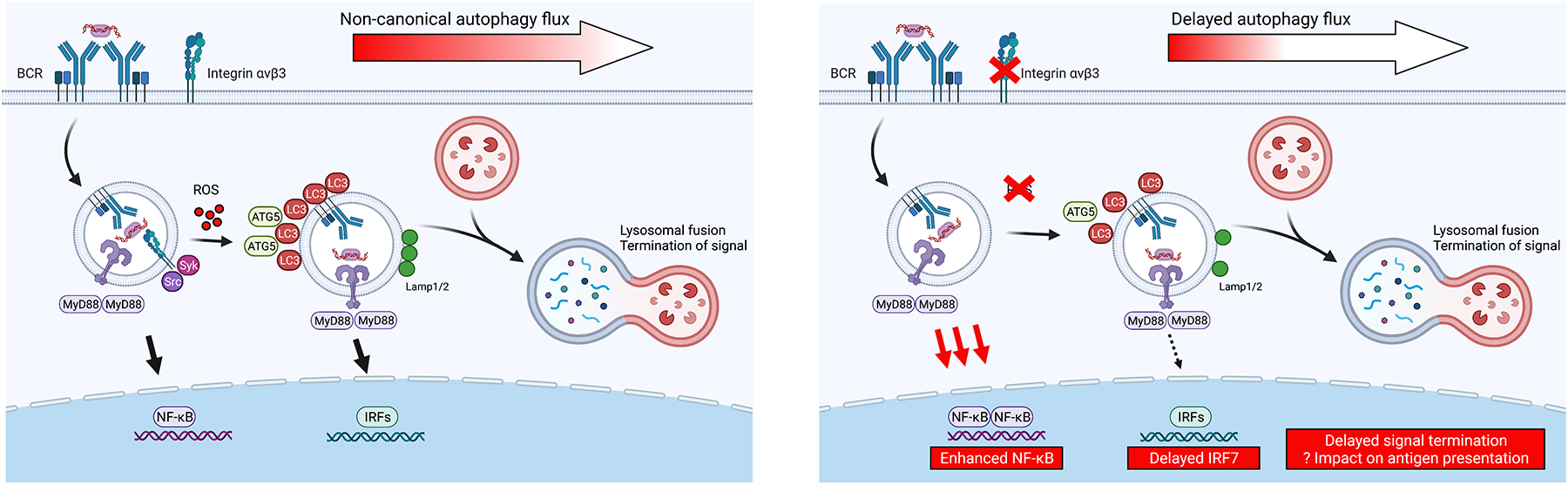
We propose a model in which variants in specific genes, such as αvβ3 integrin or non-canonical autophagy components (ATG5, ATG7, NCF1 and NCF2), result in delayed endolysosomal trafficking and consequently enhanced TLR-dependent NF-κB activation and delayed termination of TLR signaling. Left panel shows normal physiological context in which response to TLR ligands and associated antigens is limited through the integrin autophagy pathway. Right panel shows impact of lack of αvβ3 integrin or reduced NADPH oxidase-dependent ROS production which may drive enhanced NF-κB/MAPK activation and prolong TLR signals, ultimately impacting B cell proliferation, affinity maturation, and plasma cell differentiation. It remains to be determined how these changes in TLR signaling and lysosomal fusion affect processing and presentation of antigens by B cells.
