FIGURE 1.
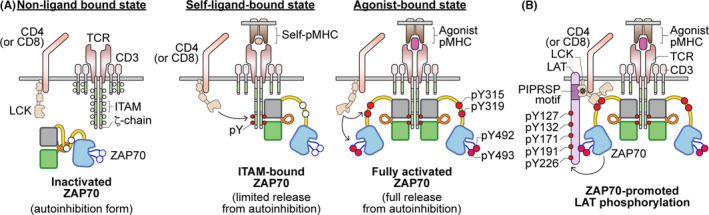
Illustration of ZAP70 activities during TCR recognition of self‐pMHC or agonist‐pMHC. (A) In the resting state, when the TCR does not interact with any pMHC, ITAMs are not phosphorylated and ZAP70 remains in an autoinhibited state in the cytoplasm. When the TCR recognizes a self‐pMHC, the interaction with self‐pMHC delivers a sufficient tonic signal to lead to ITAM phosphorylation by the kinase LCK. This then results in the recruitment of ZAP70 to the phosphorylated ITAM motifs but ZAP70 does not become phosphorylated. The degree of self‐reactivity is associated with the amount of ζ‐chain phosphorylation. Upon recognition of agonist pMHC, coreceptor‐associated LCK is brought into the vicinity of engaged TCR and CD3 complexes long enough to phosphorylate ITAMs and recruit as well as phosphorylate ZAP70 that is bound to phosphorylated‐ITAMs. Phosphorylation of ZAP70 by LCK on Y315, Y319 and Y493 leads to its activation. (B) Activated ZAP70 subsequently phosphorylates LAT on five key tyrosine residues. Notably, the SH3 domain of LCK (marked with *) interacts with the PIPRSP motif of LAT to actively recruit LAT to the activated ZAP70. This active recruitment of LAT by LCK enhances ZAP70‐mediated LAT phosphorylation and augments T cell sensitivity towards weak ligand stimuli. Furthermore, phosphorylated LAT and another ZAP70 substrate, SLP76, can function as scaffold proteins to recruit other downstream signaling proteins and events, which can eventually lead to T cell activation
