Abstract
Objective
While COVID-19 vaccination prevents severe infections, poor immunogenicity in immunocompromised people threatens vaccine effectiveness. We analysed the clinical characteristics of patients with rheumatic disease who developed breakthrough COVID-19 after vaccination against SARS-CoV-2.
Methods
We included people partially or fully vaccinated against SARS-CoV-2 who developed COVID-19 between 5 January and 30 September 2021 and were reported to the Global Rheumatology Alliance registry. Breakthrough infections were defined as occurring ≥14 days after completion of the vaccination series, specifically 14 days after the second dose in a two-dose series or 14 days after a single-dose vaccine. We analysed patients’ demographic and clinical characteristics and COVID-19 symptoms and outcomes.
Results
SARS-CoV-2 infection was reported in 197 partially or fully vaccinated people with rheumatic disease (mean age 54 years, 77% female, 56% white). The majority (n=140/197, 71%) received messenger RNA vaccines. Among the fully vaccinated (n=87), infection occurred a mean of 112 (±60) days after the second vaccine dose. Among those fully vaccinated and hospitalised (n=22, age range 36–83 years), nine had used B cell-depleting therapy (BCDT), with six as monotherapy, at the time of vaccination. Three were on mycophenolate. The majority (n=14/22, 64%) were not taking systemic glucocorticoids. Eight patients had pre-existing lung disease and five patients died.
Conclusion
More than half of fully vaccinated individuals with breakthrough infections requiring hospitalisation were on BCDT or mycophenolate. Further risk mitigation strategies are likely needed to protect this selected high-risk population.
Keywords: COVID-19, vaccination, antirheumatic agents
Key messages.
What is already known about this subject?
COVID-19 vaccination is recommended and usually well tolerated and efficacious among people with rheumatic disease.
However, laboratory-based studies using surrogate markers of protection against COVID-19 have demonstrated reduced vaccine immunogenicity, particularly humoral immunity, in people taking certain immunosuppressive medications.
What does this study add?
Among fully vaccinated individuals with rheumatic disease who developed breakthrough SARS-CoV-2 infections, over half of patients requiring hospitalisation were treated with either B cell-depleting therapy or mycophenolate.
Reassuringly, use of other immunomodulators including tumor necrosis factor (TNF) inhibitors was infrequent in this series.
These data provide real-world evidence, corroborating results from laboratory-based studies of surrogate correlates of protection against COVID-19.
How might this impact on clinical practice or further developments?
Breakthrough SARS-CoV-2 infection following vaccination is an ongoing concern for people with rheumatic diseases.
These data add to the knowledge about which patients are at risk of vaccine failure and strengthen the rationale for additional vaccine doses and for secondary prevention of severe COVID-19 with monoclonal antibodies and other therapies.
Introduction
Despite the established efficacy of COVID-19 vaccines, breakthrough infections still occur in those who are vaccinated.1–3 There is particular concern for people on immunomodulatory and immunosuppressive medications, including those with rheumatic disease. Studies have shown that specific classes of medications (B cell-depleting therapy (BCDT), antimetabolites and glucocorticoids) can severely hamper the humoral response and have some impact on the T cell-mediated response.4–6 Due to accumulating data demonstrating reduced immune responses in some immunosuppressed individuals, several countries have amended vaccination programmes to offer an additional dose after completion of the primary vaccine series in this population.7–12
Despite laboratory data regarding diminished antibody responses to vaccination, clinical data on breakthrough infections in people with rheumatic disease are sparse. Such data are important both to prioritise patient groups for additional vaccine doses and for guidance about use of other strategies, such as monoclonal antibodies or emerging antivirals against SARS-CoV-2, for postexposure prophylaxis or early treatment to prevent progression to severe COVID-19.
Given the need for data to inform public health measures and for counselling and care of immunocompromised patients in the clinical setting, we analysed the characteristics of people with rheumatic disease who developed COVID-19 following vaccination using the COVID-19 Global Rheumatology Alliance (C19-GRA) registry.
Methods
The C19-GRA registry was launched on 24 March 2020 and allows healthcare providers globally to enter data on people with rheumatic disease diagnosed with COVID-19 via a REDCap survey.13 14 Registry data elements collected include provider name, city, country and clinic, and patient age, sex, race and ethnicity. Data include rheumatic disease medications, physician global assessment of disease activity (remission, low, moderate or high) and comorbidities at the time of COVID-19 diagnosis. We also included information on whether medications were held in online supplemental table 3. Data on COVID-19 include diagnosis date, symptoms, treatments and outcomes, with available laboratory results also collected.
rmdopen-2021-002187supp001.pdf (54.1KB, pdf)
On 5 January 2021, an initial set of vaccine-related questions were added to the registry, including whether patients had received a COVID-19 vaccine, which vaccine was received, how many doses and date of the most recent dose. Additional questions, related to timing of infection and specific rheumatic disease medications at the time of vaccination and whether they were held with each vaccine dose, were added on 8 July 2021. This study reports on people with breakthrough SARS-CoV-2 infection following vaccination who were entered into the registry between 5 January 2021 and 30 September 2021. The current analysis includes previously published cases from Lawson-Tovey et al15 (n=8) and Cook et al16 (n=16).
We analysed SARS-CoV-2 infection following vaccination reported to the registry, with a particular focus on individuals who were fully vaccinated, especially with regard to hospitalisation. We defined ‘partially vaccinated’ as being ≤14 days after the first dose in a two-dose series or within 13 days of a single-dose vaccine.17 Breakthrough infection among fully vaccinated individuals was defined according to the US Centers for Disease Control and Prevention (CDC) as infection occurring ≥14 days after the second dose in a two-dose series or ≥14 days after a single-dose vaccine.17 We excluded people with COVID-19 who were within 14 days of their first dose of a two-dose series (n=25) as the CDC definition considers these individuals to be unvaccinated. Continuous variables are reported as mean (SD). Categorical variables are reported as number and percentage. We used a histogram to visually assess time from vaccination to infection.
Patient and public involvement
As members of the C19-GRA, including its Steering Committee and Patient Board, patients were involved in the design, conduct, reporting or dissemination plans of this research.
Results
We identified 110 partially and 87 fully vaccinated patients with rheumatic disease in the C19-GRA registry. Demographic and clinical characteristics of fully vaccinated individuals are shown in table 1; partially vaccinated individuals are described in online supplemental table 1. Fully vaccinated individuals (n=87) had a mean age of 54 years, and 77% were female and 56% were white. The majority (75%) were from North America. The most common rheumatic diseases were rheumatoid arthritis (39%), psoriatic arthritis (14%) and systemic lupus erythematosus (12%). At the time of infection, 34% were taking conventional synthetic disease-modifying antirheumatic drugs only, 28% biologic/targeted synthetic disease-modifying antirheumatic drugs only and 31% were on both; 7% of patients were not taking any disease-modifying antirheumatic drug. The majority (70%) were not on glucocorticoids; among those taking glucocorticoids, 21% were taking prednisone 1–9 mg/day and 7% were on ≥10 mg/day. The majority (79%) had physician-reported remission or low disease activity at the time of breakthrough infection; 21% had moderate or high disease activity. The most common comorbidities were hypertension (28%), obesity (21%), lung disease (18%) and diabetes (10%); 47% had one or more comorbidities. The majority received messenger RNA (mRNA) vaccines (Pfizer n=45, 52%; Moderna n=21, 24%).
Table 1.
Demographic and disease characteristics of fully vaccinated* individuals with rheumatic disease diagnosed with SARS-CoV-2 infection after vaccination reported to the C19-GRA registry (n=87)
| Frequency (%) or mean (SD) | |
| Mean age (years), SD | 53.8 (16.3) |
| Female | 67 (77) |
| Race or ethnicity | |
| White | 49 (56.3) |
| Black | 6 (6.9) |
| Latin American | 10 (11.5) |
| East or South Asian | 7 (8.1) |
| Other | 9 (10.3) |
| Unknown | 6 (6.9) |
| WHO regions | |
| African region | 2 (2.3) |
| Region of the Americas - North | 65 (74.7) |
| Region of the Americas - South | 1 (1.2) |
| South-East Asian region | 0 (0) |
| European region | 8 (9.2) |
| Eastern Mediterranean region | 7 (8.1) |
| Western Pacific region | 4 (4.6) |
| Rheumatic disease† | |
| Rheumatoid arthritis | 34 (39.1) |
| Systemic lupus erythematosus | 10 (11.5) |
| Psoriatic arthritis | 12 (13.8) |
| Vasculitis | 10 (11.5) |
| Inflammatory myopathy | 8 (9.2) |
| Spondyloarthritis (axial and other) | 3 (3.5) |
| Sjogren’s syndrome | 4 (4.6) |
| Systemic sclerosis | 4 (4.6) |
| Other‡ | 8 (9.2) |
| Comorbidity count | |
| 0 | 46 (52.9) |
| 1 | 26 (29.9) |
| ≥2 | 15 (17.2) |
| Most common comorbidities | |
| Hypertension | 24 (27.6) |
| Obesity | 18 (20.7) |
| Lung disease | 16 (18.4) |
| Diabetes | 9 (10.3) |
| Chronic kidney disease | 8 (9.2) |
| Medication prior to COVID-19 diagnosis§ | |
| No DMARD | 6 (6.9) |
| csDMARDs | 57 (65.5) |
| Methotrexate | 21 (24.1) |
| Hydroxychloroquine | 25 (28.7) |
| Leflunomide | 6 (6.9) |
| Azathioprine | 6 (6.9) |
| Mycophenolate | 10 (11.5) |
| Sulfasalazine | 4 (4.6) |
| Colchicine | 2 (2.3) |
| b/tsDMARDs | 51 (58.6) |
| B cell-depleting therapy | 16 (18.4) |
| TNF inhibitors | 19 (21.8) |
| Other biologics¶ | 10 (11.5) |
| JAK inhibitors | 6 (6.9) |
| Glucocorticoid dose | |
| 0 mg/day | 61 (70.1) |
| 1–9 mg/day | 18 (20.7) |
| ≥10 mg/day | 6 (6.9) |
| Missing/unknown | 2 (2.3) |
| Disease activity | |
| Remission/low | 69 (79.3) |
| Moderate/high | 18 (20.7) |
| Confirmed COVID-19** | 87 (100) |
| Vaccine | |
| Pfizer-BioNTech | 45 (51.7) |
| Moderna | 21 (24.1) |
| AstraZeneca/Oxford | 6 (6.9) |
| Sinovac | 5 (5.7) |
| Janssen/Johnson & Johnson | 6 (6.9) |
| Don’t know/missing | 4 (4.6) |
*Fully vaccinated: infection ≥14 days after second dose of a two-dose vaccine or first if Janssen/Johnson & Johnson.
†Cases could have more than one disease diagnosis.
‡Other rheumatic diseases include mixed connective tissue (n=2), antiphospholipid antibody syndrome (n=1), autoinflammatory syndrome (n=1), IgG4-related disease (n=1), undifferentiated connective tissue disease (n=1), Still’s disease (n=1) and palindromic rheumatism (n=1).
§csDMARD medications included antimalarials (hydroxychloroquine, chloroquine), azathioprine, cyclophosphamide, ciclosporin, leflunomide, methotrexate, mycophenolate mofetil/mycophenolic acid, sulfasalazine and tacrolimus; b/tsDMARD included abatacept, belimumab, CD20 inhibitors, IL-1 inhibitors, IL-6 inhibitors, IL-12/23 inhibitors, IL-17 inhibitors, anti-TNF and Janus kinase inhibitors.
¶Other biologics include abatacept (n=4), IL-6 (n=2), IL-1 (n=2), belimumab (n=1) and ustekinumab (n=1).
**Confirmed COVID-19 diagnosis: diagnosis made via PCR, antigen or antibody test.
††
BMI, body mass index; b/tsDMARD, biologic/targeted synthetic disease-modifying antirheumatic drugs; C19-GRA, COVID-19 Global Rheumatology Alliance; csDMARD, conventional synthetic disease-modifying antirheumatic drugs; DMARD, disease-modifying antirheumatic drugs; JAK, Janus kinase; TNF, tumor necrosis factor.
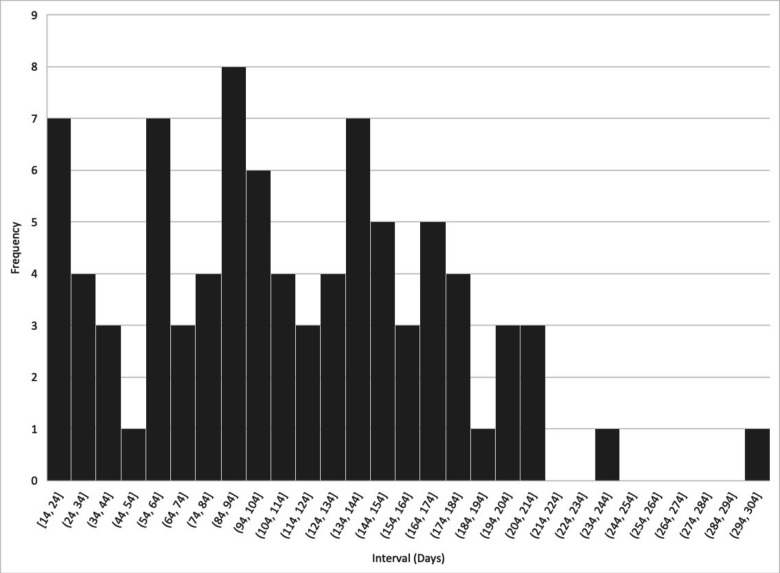
Among the fully vaccinated, infection occurred at a mean of 112 (±60, range 14–300) days after the second dose (figure 1) and 26% were hospitalised. The most common COVID-19 symptoms were cough (69%), fever (58%), malaise (52%), myalgia (39%) and shortness of breath (37%) (table 2). There were relatively few COVID-19 complications reported: three patients experienced acute respiratory distress syndrome (4%), five had a concomitant or secondary infection (three with pneumonia, one secondary sinus infection, one acute kidney injury; 6%), three patients experienced sepsis (4%) and no patients had cytokine storm reported.
Figure 1.
Number of days between last vaccination and SARS-CoV-2 infection among fully vaccinated individuals in the COVID-19 Global Rheumatology Alliance registry. The x-axis represents the interval of days during which infection occurred after the final dose of vaccine.
Table 2.
COVID-19 symptoms and outcomes in fully vaccinated individuals with rheumatic disease who were vaccinated reported to the C19-GRA registry
| Most frequent reported symptoms | n (%) |
| Cough | 60 (69.0) |
| Fever | 50 (57.5) |
| Malaise | 45 (51.7) |
| Myalgia | 34 (39.1) |
| Shortness of breath | 32 (36.8) |
| COVID-19 complications | |
| ARDS | 3 (3.5) |
| Sepsis | 3 (3.5) |
| Concomitant or secondary infection | 5 (5.8) |
| Cytokine storm (or MAS) | 0 (0) |
| Outcomes | |
| Hospitalised (n=86*) | 22 (26) |
| Death | 5 (6) |
*1 unknown status.
ARDS, acute respiratory distress syndrome; C19-GRA, COVID-19 Global Rheumatology Alliance; MAS, macrophage activation syndrome.
Medications at the time of COVID-19 diagnosis are reported in online supplemental table 2 for the full cohort (n=197), for the fully vaccinated (n=87) and for those hospitalised among the fully vaccinated (n=22). Among the fully vaccinated, 24% were on methotrexate, compared with 9% of those who were both fully vaccinated and hospitalised. A similar pattern was seen for tumor necrosis factor (TNF) inhibitors (22% fully vaccinated vs 9% fully vaccinated and hospitalised). In contrast, 18% of those fully vaccinated were on BCDT, compared with 46% of those fully vaccinated and hospitalised. Among the fully vaccinated and among the fully vaccinated and hospitalised, the majority were not taking systemic glucocorticoids at the time of vaccination (72% and 64%, respectively).
Among 79 fully vaccinated individuals with information on medication status at the time of vaccination, all but seven continued their antirheumatic medications before their vaccine doses (online supplemental table 3). Five discontinued medications after their vaccine doses. Otherwise medications were similar to those at the time of COVID-19 diagnosis.
Of those who were considered fully vaccinated, 22 were hospitalised (table 3). At the time of diagnosis, nine were being treated with BCDT, six as monotherapy and three in combination with other immunosuppressive medications. Three were on mycophenolate and three were on azathioprine. Among 17 individuals who had information on holding medications at the time of vaccination, only one individual withheld medications. Eleven received the Pfizer vaccine, five received Moderna, and two each received Janssen/Johnson & Johnson and Oxford/AstraZeneca. The median time from vaccination to COVID-19 diagnosis was 59 days (range 14–180 days). The four patients who required invasive ventilation subsequently died, and one patient who received non-invasive ventilation also died. Among the five deaths, one individual was aged 41–50, three individuals were aged 61–70 and one was over 80 years. Three individuals who died were on BCDT at the time of vaccination.
Table 3.
Details of fully vaccinated and hospitalised individuals reported to the C19-GRA registry (n=22)
| Age and sex | Comorbidities | Rheumatic disease | Medications at the time of vaccination | Medications held for vaccination | Medications at the time of COVID-19 diagnosis | Vaccine received, time from last vaccination to SARS-CoV-2 infection | Outcome of hospitalisation* |
| 31–40, F | None | Sjogren’s | Hydroxychloroquine, methotrexate, BCDT | Unknown, B cell depletion unknown | Hydroxychloroquine, methotrexate, BCDT | Pfizer-BioNTech, 61 days | No supplemental oxygen |
| 31–40, F | Lung disease, diabetes, chronic neurological/neuromuscular disease | SLE | Belimumab, mycophenolate | No | Belimumab, mycophenolate | Moderna, 23 days | No supplemental oxygen |
| 31–40, F | Hypertension, BMI ≥30 | Inflammatory myopathy | Leflunomide, BCDT, glucocorticoid | No, not B cell-depleted | Leflunomide, BCDT, glucocorticoid | Unknown, 30 days | Supplemental oxygen |
| 31–40, F | None | Psoriatic arthritis | None | – | TNFi | Pfizer-BioNTech, 170 days | No supplemental oxygen |
| 41–50, M | Hypertension | Psoriatic arthritis | None | – | None | Janssen/Johnson & Johnson, 24 days | Supplemental oxygen |
| 41–50, F | Lung disease | RA | Azathioprine | Unknown | Azathioprine | Pfizer-BioNTech, 55 days | Supplemental oxygen |
| 41–50, F | Lung disease, BMI ≥30, kidney disease | RA | Hydroxychloroquine, glucocorticoid | No | TNFi, hydroxychloroquine, glucocorticoid | Unknown, 120 days | Invasive ventilation/ECMO, death |
| 41–50, F | Hypertension, kidney disease, organ transplant recipient, immunodeficiency, BMI >30 | SLE | Mycophenolate, glucocorticoid | No | Mycophenolate, glucocorticoid | Pfizer-BioNTech, 14 days | Supplemental oxygen |
| 51–60, F | Hypertension | RA | IL-6 inhibitor | Unknown | IL-6 inhibitor | AstraZeneca/Oxford, 30 days | Supplemental oxygen |
| 61–70, M | Diabetes | Inflammatory myopathy | Glucocorticoid | No | BCDT, glucocorticoid | Pfizer-BioNTech, 180 days | Invasive ventilation/ECMO, death |
| 61–70, M | Lung disease, hypertension, cardiovascular disease | Axial spondyloarthritis | BCDT | B cell-depleted | BCDT | Pfizer-BioNTech, 57 days | Non-invasive ventilation or high-flow oxygen devices, death |
| 61–70, M | Lung disease, hypertension, cardiovascular disease, kidney disease | ANCA-associated vasculitis | BCDT | B cell-depleted | BCDT | Moderna, 14 days | Supplemental oxygen |
| 61–70, F | Lung disease | RA | BCDT, glucocorticoid | GC: no, B cell-depleted | BCDT, glucocorticoid | Moderna, 78 days | Invasive ventilation, death |
| 61–70, F | None | RA | Abatacept | No | Abatacept | AstraZeneca/Oxford, 65 days | Discharged from hospital (no ventilation reported) |
| 61–70, F | Diabetes, BMI ≥30, hypertension, cardiovascular disease, kidney disease | Vasculitis | Glucocorticoid | No | Glucocorticoid | Pfizer-BioNTech, 150 days | Supplemental oxygen |
| 61–70, F | None | RA | None | – | Methotrexate, JAKi | Pfizer-BioNTech, 54 days | Supplemental oxygen |
| 61–70, F | None | Systemic sclerosis, inflammatory myopathy | Azathioprine/6-MP, BCDT | B cell depletion unknown | Azathioprine/6-MP, BCDT | Moderna, 16 days | Discharged from hospital (no ventilation reported) |
| 71–80, M | Hypertension, cardiovascular disease, kidney disease | Inflammatory myopathy | Mycophenolate | Unknown | Mycophenolate | Pfizer-BioNTech, 173 days | Supplemental oxygen |
| 71–80, F | Lung disease | RA | BCDT | B cell-depleted | BCDT | Janssen/Johnson & Johnson, 38 days | Supplemental oxygen |
| >80, M | Lung disease, hypertension, cardiovascular disease | Vasculitis | BCDT, glucocorticoid | No, B cell depletion unknown | BCDT, glucocorticoid | Pfizer-BioNTech, 100 days | Invasive ventilation/ECMO, death |
| >80, M | Cardiovascular disease, cancer | Psoriatic arthritis | Glucocorticoid | Yes | Ustekinumab, glucocorticoid | Pfizer-BioNTech, 140 days | Non-invasive ventilation or high-flow oxygen devices |
| >80, M | Hypertension, kidney disease | Vasculitis | BCDT | B cell depletion unknown | BCDT | Moderna, 180 days | Non-invasive ventilation or high-flow oxygen devices |
*Highest level of hospital treatment; if no discharge status, they were alive at discharge.
ANCA, Antineutrophil cytoplasmic antibody; BCDT, B cell-depleting therapy; BMI, body mass index; C19-GRA, COVID-19 Global Rheumatology Alliance; ECMO, extracorporeal membrane oxygenation; F, female; GC, glucocorticoid; IL, interleukin; JAKi, Janus kinase inhibitor; M, male; 6-MP, 6-mercaptopurine; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; TNFi, tumor necrosis factor inhibitor.
Discussion
We found that over half of fully vaccinated individuals with rheumatic disease with breakthrough SARS-CoV-2 infections requiring hospitalisation had been taking either BCDT or mycophenolate at the time of COVID-19 diagnosis. Furthermore, we did not find any meaningful differences by hospitalisation status in glucocorticoid use among those with breakthrough infections. Reassuringly, breakthrough infections leading to hospitalisation were infrequent among those using other immunomodulators, including TNF inhibitors, corroborating findings from multiple registries.18
Despite the demonstrated efficacy of COVID-19 vaccines, particularly mRNA platform vaccines, breakthrough infections occurred in the fully vaccinated even prior to the emergence of more transmissible variants of concern.1–3 Cook et al16 reported a case series of 16 patients with rheumatic disease with breakthrough infections from a single healthcare system in Massachusetts, of whom 6 were hospitalised and 2 died. In the EULAR registries of breakthrough infections in patients with rheumatic disease, 28 individuals were fully vaccinated; 74% fully recovered while 2 died.15 A limitation of both our study and prior studies is the inability to confirm denominators for these populations of interest and thus we cannot estimate the incidence of SARS-CoV-2 infection following vaccination.
The impact on vaccine immunogenicity from medications used for rheumatic disease has been studied using surrogates for protection for humoral and T cell-mediated responses. In the general population, antibody neutralisation titres have correlated well with clinical protection against COVID-19.19 Overall, antibody titres have been lower among those with rheumatic disease and on immunosuppressive or immunomodulatory medications compared with healthy controls, particularly for those on BCDT such as rituximab or mycophenolate.4 5 20–28 In addition, several case series and cohort studies of people on rituximab showed that undetectable CD19-positive cells correlate with the lack of seroconversion, although this did not appear to affect the T cell response.6 19 20 25 29–32 The precise clinical implications of these lower antibody responses in conjunction with maintained T cell responses are still unclear.
Clinical data documenting the characteristics of rheumatology patients with breakthrough severe COVID-19 have been limited. In our study, 9 out of 22 fully vaccinated individuals hospitalised for breakthrough infections were treated with BCDT (41%), compared with 11% of individuals with infections after partial or full vaccination overall and 4% of the entire GRA registry as of 30 September 2021. Monotherapy or combination therapy with mycophenolate was also over-represented among those hospitalised for breakthrough infections, although less frequently than BCDT. Reassuringly, cases of hospitalisation were infrequent in patients taking commonly prescribed medications like methotrexate and TNF inhibitors. Thus our findings, with real-world clinical outcomes, support the inferences drawn from prior studies that have used surrogates for protection.
There is a lack of data regarding comparative effectiveness between vaccine types in this population. In a cohort study of responses to Janssen/Johnson & Johnson versus mRNA vaccines among individuals with rheumatic disease, there were lower odds of seroconversion with the former.33 Due to the nature of our study design and small numbers, we were unable to directly compare the efficacy of specific vaccines in the rheumatic disease population.
Due to concerns of lower efficacy of vaccination among the immunocompromised, additional doses of vaccine (typically third doses of an mRNA vaccine) have been studied among organ transplant recipients and haemodialysis patients and found to be safe and effective in increasing antibody levels.34–38 Improved humoral responses following a third vaccine dose have also been reported in people with rheumatoid arthritis39 40 and in a case series of 18 individuals with rheumatic disease.41 Multiple countries approved additional vaccine doses in the immunocompromised, including the UK in July and the USA in August 2021, before these were approved for the general population.7 While our study does not include data on breakthrough infection after an additional or third dose, the overall evidence has suggested that an additional dose is especially warranted in high-risk patients on certain immunosuppressive or immunomodulatory medications. Further studies reporting breakthrough infections in those with a third or fourth vaccine dose will help inform the effectiveness of this strategy.
The current totality of evidence4 5 20–25 supports the need to improve monoclonal antibody access42 43 for the most vulnerable patients who may not mount an adequate response following vaccination. In addition, further studies about passive immunity or pre-exposure prophylaxis are needed. Finally, new oral antiviral therapies may be potential options for administration in an outpatient setting, but more research on efficacy in people with rheumatic diseases or immunosuppressed populations is needed.44 45
The strengths of this study include using a large global registry to collect data on breakthrough infections among people with rheumatic disease who have been vaccinated. However, limitations of our study must be acknowledged. First, there is potential for selection bias in this voluntary registry, particularly over-representation of those at highest risk of poor vaccine responses and thus breakthrough infections. Incidence rates, including mortality rates, cannot be reliably estimated using these data due to the lack of clear denominators for this population. Second, this study was cross-sectional, and although we assessed the timing of infection and medication holding with respect to the timing of vaccine doses, our study did not include biospecimen collection to measure antibody titres or other surrogate measures of protection. Finally, although this case series is relatively large, the study design and small numbers within categories preclude assessing differences between rheumatic diseases, medication classes and vaccine types. We intentionally present descriptive data due to the lack of clear denominators and comparator group; as outlined in a recent paper, descriptive work is often harmed by inappropriate statistical adjustment or other statistical testing.46 Given the descriptive nature of this work and the potential sources of bias, results should be interpreted with caution and studies with appropriate denominators (eg, prospective cohort studies) are necessary to confirm our results.
Conclusion
We present the largest series to date of breakthrough COVID-19 among people with rheumatic disease. Our data support prior findings of reduced vaccine immunogenicity based on the use of certain classes of antirheumatic medications. Given the high frequency of people with rheumatic disease on medications such as BCDT and mycophenolate who required hospitalisation, these patients should be prioritised and strongly recommended for other risk mitigation measures beyond additional doses of vaccine. Moreover, the current evidence supports the use of strategies that compensate for a reduced or absent humoral immune response to vaccination in high-risk individuals with rheumatic diseases, such as additional vaccine doses or pre-exposure or postexposure prophylaxis with monoclonal antibodies.
Footnotes
Twitter: @rheum_cat, @carmona_loreto, @pedrommcmachado, @philipcrobinson
JL and MG contributed equally.
Contributors: All authors contributed to the study design, data collection, interpretation of results and review/approval of the final submitted manuscript. JL and MG are guarantors for this manuscript.
Funding: MG reports grants from the National Institutes of Health, NIAMS, outside the submitted work. KLH is supported by the NIHR Manchester Biomedical Research Centre. JS is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers: R01 AR077607, P30 AR070253 and P30 AR072577), and the R Bruce and Joan M Mickey Research Scholar Fund. JH is supported by grants from the Rheumatology Research Foundation. ZW is supported by grants from the National Institutes of Health. PMM is supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC). JY is supported by grants from the National Institutes of Health (K24 AR074534 and P30 AR070155).
Disclaimer: The views expressed here are those of the authors and participating members of the COVID-19 Global Rheumatology Alliance and do not necessarily represent the views of the American College of Rheumatology (ACR), EULAR, the UK National Health Service (NHS), the National Institute for Health Research (NIHR), the UK Department of Health or any other organisation.
Competing interests: KLH reports she has received non-personal speaker’s fees from AbbVie and grant income from BMS, UCB and Pfizer, all unrelated to this manuscript; KLH is supported by the NIHR Manchester Biomedical Research Centre. LG reports personal consultant fees from AbbVie, Amgen, BMS, Biogen, Celgene, Gilead, Janssen, Lilly, Novartis, Pfizer, Samsung Bioepis, Sanofi-Aventis and UCB, and grants from Amgen, Lilly, Janssen, Pfizer, Sandoz, Sanofi and Galapagos, all unrelated to this manuscript. AS reports research grants from a consortium of 14 companies (among them AbbVie, BMS, Celltrion, Fresenius Kabi, Gilead/Galapagos, Lilly, Mylan/Viatris, Hexal, MSD, Pfizer, Roche, Samsung, Sanofi-Aventis and UCB) supporting the German RABBIT register and personal fees from lectures for AbbVie, MSD, Roche, BMS, Lilly and Pfizer, all unrelated to this manuscript. LC has not received fees or personal grants from any laboratory, but her institute works by contract for laboratories among other institutions, such as AbbVie Spain, Eisai, Gebro Pharma, Merck Sharp & Dohme España, Novartis Farmaceutica, Pfizer, Roche Farma, Sanofi-Aventis, Astellas Pharma, Actelion Pharmaceuticals España, Grünenthal and UCB Pharma. EF-M reports personal consultant fees from Boehringer Ingelheim Portugal and that LPCDR received support for specific activities: grants from AbbVie, Novartis, Janssen-Cilag, Lilly Portugal, Sanofi, Grünenthal, MSD, Celgene, Medac, Pharmakern and GAfPA; grants and non-financial support from Pfizer; and non-financial support from Grünenthal, outside the submitted work. IB reports personal consultant fees from AbbVie, Novartis, Pfizer and Janssen, all unrelated to this manuscript. JZ reports speaker fees from AbbVie, Novartis and Janssen/Johnson & Johnson, all unrelated to this manuscript. GR-C reports personal consultant fees from Eli Lilly and Novartis, all unrelated to this manuscript. JS is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant numbers: R01 AR077607, P30 AR070253 and P30 AR072577), and the R Bruce and Joan M Mickey Research Scholar Fund. JS has received research support from Amgen and Bristol Myers Squibb and performed consultancy for Bristol Myers Squibb, Gilead, Inova, Janssen and Optum, unrelated to this work. LW receives speaker’s bureau fees from Aurinia Pharma, unrelated to this manuscript. SB reports no competing interests related to this work. He reports non-branded consulting fees for AbbVie, Horizon and Novartis (all <$10 000). MGM has no competing interests related to this work. She serves as a patient consultant for BMS, BI JNJ and Aurinia (all <$10 000). RG reports no competing interests related to this work. Outside of this work she reports personal and/or speaking fees from AbbVie, Janssen, Novartis, Pfizer and Cornerstones and travel assistance from Pfizer (all <$10 000). JH reports no competing interests related to this work. He is supported by grants from the Rheumatology Research Foundation and has salary support from the Childhood Arthritis and Rheumatology Research Alliance. He has performed consulting for Novartis, Sobi and Biogen, all unrelated to this work (<$10 000). ESi reports non-financial support from Canadian Arthritis Patient Alliance, outside the submitted work. PS reports personal fees from the American College of Rheumatology/Wiley Publishing, outside the submitted work. ZW reports grant support from Bristol Myers Squibb and Principia/Sanofi and performed consultancy for Viela Bio and MedPace, outside the submitted work. His work is supported by grants from the National Institutes of Health. PMM has received consulting/speaker’s fees from AbbVie, BMS, Celgene, Eli Lilly, Galapagos, Janssen, MSD, Novartis, Orphazyme, Pfizer, Roche and UCB, all unrelated to this study. PMM is supported by the National Institute for Health Research (NIHR) University College London Hospitals (UCLH) Biomedical Research Centre (BRC). PCR reports no competing interests related to this work. Outside of this work PCR reports personal fees from AbbVie, Atom Bioscience, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Kukdong, Novartis, UCB, Roche and Pfizer; meeting attendance support from BMS, Pfizer and UCB; and grant funding from Janssen, Novartis, Pfizer and UCB Pharma (all <$10 000). JY reports no competing interests related to this work. Her work is supported by grants from the National Institutes of Health (K24 AR074534 and P30 AR070155). Outside of this work, she has received research grants or performed consulting for Gilead, BMS Foundation, Pfizer, Aurinia and AstraZeneca.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Researchers interested in performing additional analyses from survey data are invited to submit proposals through the COVID-19 Global Rheumatology Alliance at rheum-covid.org. For approved projects, we will be able to provide summary tables and data analyses as requested. We do not currently have IRB approval to make the raw data available to other researchers.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
The C19-GRA physician registry was determined to be 'not human subjects research' under US federal guidelines assessed by the University of California, San Francisco, and patient consent was therefore not required.
References
- 1.Brosh-Nissimov T, Orenbuch-Harroch E, Chowers M, et al. BNT162b2 vaccine breakthrough: clinical characteristics of 152 fully vaccinated hospitalized COVID-19 patients in Israel. Clin Microbiol Infect 2021;27:1652–7. 10.1016/j.cmi.2021.06.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med 2021;385:1474–84. 10.1056/NEJMoa2109072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown CM, Vostok J, Johnson H, et al. Outbreak of SARS-CoV-2 Infections, Including COVID-19 Vaccine Breakthrough Infections, Associated with Large Public Gatherings - Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep 2021;70:1059–62. 10.15585/mmwr.mm7031e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deepak P, Kim W, Paley MA, et al. Effect of Immunosuppression on the Immunogenicity of mRNA Vaccines to SARS-CoV-2 : A Prospective Cohort Study. Ann Intern Med 2021;174:1572–85. 10.7326/M21-1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haberman RH, Herati R, Simon D, et al. Methotrexate hampers immunogenicity to BNT162b2 mRNA COVID-19 vaccine in immune-mediated inflammatory disease. Ann Rheum Dis 2021;80:1339–44. 10.1136/annrheumdis-2021-220597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kearns P, Siebert S, Willicombe M. Examining the immunological effects of COVID-19 vaccination in patients with conditions potentially leading to diminished immune response capacity – the OCTAVE trial 2021. Lancet 2021. [Google Scholar]
- 7.United States food and drug administration. coronavirus (COVID-19) update: FDA Authorizes additional vaccine dose for certain immunocompromised individuals. United States food and drug administration. Available: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised [Accessed 9 Sep 2021].
- 8.Israeli Councils For Health . Summary of an urgent meeting on the issue of vaccines against corona for those who have been vaccinated. Israeli councils for health. Available: https://govextra.gov.il/media/30095/meeting-summary-15122020.pdf [Accessed 9 Sep 2021].
- 9.COVID-19 vaccination programme information for healthcare practitioners. public health England. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1009174/COVID-19_vaccination_programme_guidance_for_healthcare_workers_6_August_2021_v3.10.pdf [Accessed 9 Sep 2021].
- 10.Die wichtigsten Fragen und Antworten Zur Corona-Impfung. services Der BUNDESREGIERUNG. Available: https://www.bundesregierung.de/breg-de/themen/coronavirus/coronavirus-impfung-faq-1788988 [Accessed 9 Sep 2021].
- 11.Landewé RB, Machado PM, Kroon F, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis 2020;79:851–8. 10.1136/annrheumdis-2020-217877 [DOI] [PubMed] [Google Scholar]
- 12.Friedman MA, Curtis JR, Winthrop KL. Impact of disease-modifying antirheumatic drugs on vaccine immunogenicity in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1255–65. 10.1136/annrheumdis-2021-221244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liew JW, Bhana S, Costello W, et al. The COVID-19 global rheumatology alliance: evaluating the rapid design and implementation of an international registry against best practice. Rheumatology 2021;60:353–8. 10.1093/rheumatology/keaa483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallace ZS, Bhana S, Hausmann JS, et al. The rheumatology community responds to the COVID-19 pandemic: the establishment of the COVID-19 global rheumatology alliance. Rheumatology 2020;59:1204–6. 10.1093/rheumatology/keaa191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lawson-Tovey S, Hyrich KL, Gossec L. SARS-CoV-2 infection after vaccination in patients with inflammatory rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021. 10.1136/annrheumdis-2021-221217 [DOI] [PubMed] [Google Scholar]
- 16.Cook C, Patel NJ, D'Silva KM, et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis 2022;81:289–91. 10.1136/annrheumdis-2021-221326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cdc COVID-19 study shows mRNA vaccines reduce risk of infection by 91 percent for fully vaccinated people, 2021. Available: https://www.cdc.gov/media/releases/2021/p0607-mrna-reduce-risks.html [Accessed 28 Oct 2021].
- 18.Izadi Z, Brenner EJ, Mahil SK, et al. Association between tumor necrosis factor inhibitors and the risk of hospitalization or death among patients with immune-mediated inflammatory disease and COVID-19. JAMA Netw Open 2021;4:e2129639. 10.1001/jamanetworkopen.2021.29639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021;27:1205–11. 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 20.Moor MB, Suter-Riniker F, Horn MP, et al. Humoral and cellular responses to mRNA vaccines against SARS-CoV-2 in patients with a history of CD20 B-cell-depleting therapy (RituxiVac): an investigator-initiated, single-centre, open-label study. Lancet Rheumatol 2021;3:e789–97. 10.1016/S2665-9913(21)00251-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon D, Tascilar K, Schmidt K, et al. Humoral and cellular immune responses to SARS-CoV-2 infection and vaccination in autoimmune disease patients with B cell depletion. Arthritis Rheumatol 2022;74:33-37. 10.1002/art.41914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahil SK, Bechman K, Raharja A, et al. The effect of methotrexate and targeted immunosuppression on humoral and cellular immune responses to the COVID-19 vaccine BNT162b2: a cohort study. Lancet Rheumatol 2021;3:e627–37. 10.1016/S2665-9913(21)00212-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furer V, Eviatar T, Zisman D, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in adult patients with autoimmune inflammatory rheumatic diseases and in the general population: a multicentre study. Ann Rheum Dis 2021;80:1330–8. 10.1136/annrheumdis-2021-220647 [DOI] [PubMed] [Google Scholar]
- 24.Geisen UM, Berner DK, Tran F, et al. Immunogenicity and safety of anti-SARS-CoV-2 mRNA vaccines in patients with chronic inflammatory conditions and immunosuppressive therapy in a monocentric cohort. Ann Rheum Dis 2021;80:1306–11. 10.1136/annrheumdis-2021-220272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izmirly PM, Kim MY, Samanovic M, et al. Evaluation of immune response and disease status in systemic lupus erythematosus patients following SARS-CoV-2 vaccination. Arthritis Rheumatol 2022;74:284–94. 10.1002/art.41937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prendecki M, Clarke C, Edwards H, et al. Humoral and T-cell responses to SARS-CoV-2 vaccination in patients receiving immunosuppression. Ann Rheum Dis 2021;80:1322–9. 10.1136/annrheumdis-2021-220626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruddy JA, Connolly CM, Boyarsky BJ, et al. High antibody response to two-dose SARS-CoV-2 messenger RNA vaccination in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021;80:1351–2. 10.1136/annrheumdis-2021-220656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boyarsky BJ, Ruddy JA, Connolly CM, et al. Antibody response to a single dose of SARS-CoV-2 mRNA vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021 10.1136/annrheumdis-2021-220289. [Epub ahead of print: 23 Mar 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonelli MM, Mrak D, Perkmann T, et al. SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response. Ann Rheum Dis 2021;80:1355–6. 10.1136/annrheumdis-2021-220408 [DOI] [PubMed] [Google Scholar]
- 30.Mrak D, Tobudic S, Koblischke M, et al. SARS-CoV-2 vaccination in rituximab-treated patients: B cells promote humoral immune responses in the presence of T-cell-mediated immunity. Ann Rheum Dis 2021;80:1345–50. 10.1136/annrheumdis-2021-220781 [DOI] [PubMed] [Google Scholar]
- 31.Westhoff TH, Seibert FS, Anft M, et al. Correspondence on 'SARS-CoV-2 vaccination in rituximab-treated patients: evidence for impaired humoral but inducible cellular immune response'. Ann Rheum Dis 2021;80:e162. 10.1136/annrheumdis-2021-220756 [DOI] [PubMed] [Google Scholar]
- 32.Hadjadj J, Planas D, Ouedrani A. Immunogenicity of BNT162b2 vaccine against the alpha and delta variants in immunocompromised patients. MedRxiv 2021. 10.1101/2021.08.08.21261766 [DOI] [PubMed] [Google Scholar]
- 33.Chiang TP-Y, Connolly CM, Ruddy JA. Antibody response to the Janssen/Johnson & Johnson SARS-CoV-2 vaccine in patients with rheumatic and musculoskeletal diseases. Ann Rheum Dis 2021. 10.1136/annrheumdis-2021-221145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med Overseas Ed 2021;385:1244–6. 10.1056/NEJMc2111462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamar N, Abravanel F, Marion O, et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients. N Engl J Med 2021;385:661–2. 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benotmane I, Gautier G, Perrin P, et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA 2021. 10.1001/jama.2021.12339. [Epub ahead of print: 23 Jul 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longlune N, Nogier MB, Miedougé M, et al. High immunogenicity of a messenger RNA-based vaccine against SARS-CoV-2 in chronic dialysis patients. Nephrol Dial Transplant 2021;36:1704–9. 10.1093/ndt/gfab193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Espi M, Charmetant X, Barba T. Justification, safety, and efficacy of a third dose of mRNA vaccine in maintenance hemodialysis patients: a prospective observational study. MedRxiv 2021. 10.1101/2021.07.02.21259913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albach FN, Burmester GR, Biesen R. Successful BNT162b2 booster vaccinations in a patient with rheumatoid arthritis and initially negative antibody response. Ann Rheum Dis 2021;80:1361–2. 10.1136/annrheumdis-2021-220834 [DOI] [PubMed] [Google Scholar]
- 40.Baker MC, Mallajosyula V, Davis MM. Effective viral vector SARS-CoV-2 booster vaccination in a patient with rheumatoid arthritis after initial ineffective mRNA vaccine response. Arthritis Rheumatol 2021. 10.1002/art.41978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Connolly CM, Teles M, Frey S. Booster-dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis 2021;81. 10.1136/annrheumdis-2021-221206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody Sotrovimab. N Engl J Med 2021;385:1941–50. 10.1056/NEJMoa2107934 [DOI] [PubMed] [Google Scholar]
- 43.Evusheld (formerly AZD7442) long-acting antibody combination authorised for emergency use in the US for pre-exposure prophylaxis (prevention) of COVID-19, 2021. Available: https://www.astrazeneca.com/media-centre/press-releases/2021/evusheld-long-acting-antibody-combination-authorised-for-emergency-use-in-the-us-for-pre-exposure-prophylaxis-prevention-of-covid-19.html [Accessed 23 Jan 2022].
- 44.Jayk Bernal A, Gomes da Silva MM, Musungaie DB, et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N Engl J Med 2022;386:509–20. 10.1056/NEJMoa2116044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coronavirus (COVID-19) update: FDA Authorizes first oral antiviral for treatment of COVID-19, 2021. Available: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-first-oral-antiviral-treatment-covid-19 [Accessed 23 Jan 2022].
- 46.Conroy S, Murray EJ. Let the question determine the methods: descriptive epidemiology done right. Br J Cancer 2020;123:1351–2. 10.1038/s41416-020-1019-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2021-002187supp001.pdf (54.1KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Researchers interested in performing additional analyses from survey data are invited to submit proposals through the COVID-19 Global Rheumatology Alliance at rheum-covid.org. For approved projects, we will be able to provide summary tables and data analyses as requested. We do not currently have IRB approval to make the raw data available to other researchers.