FIG 1.
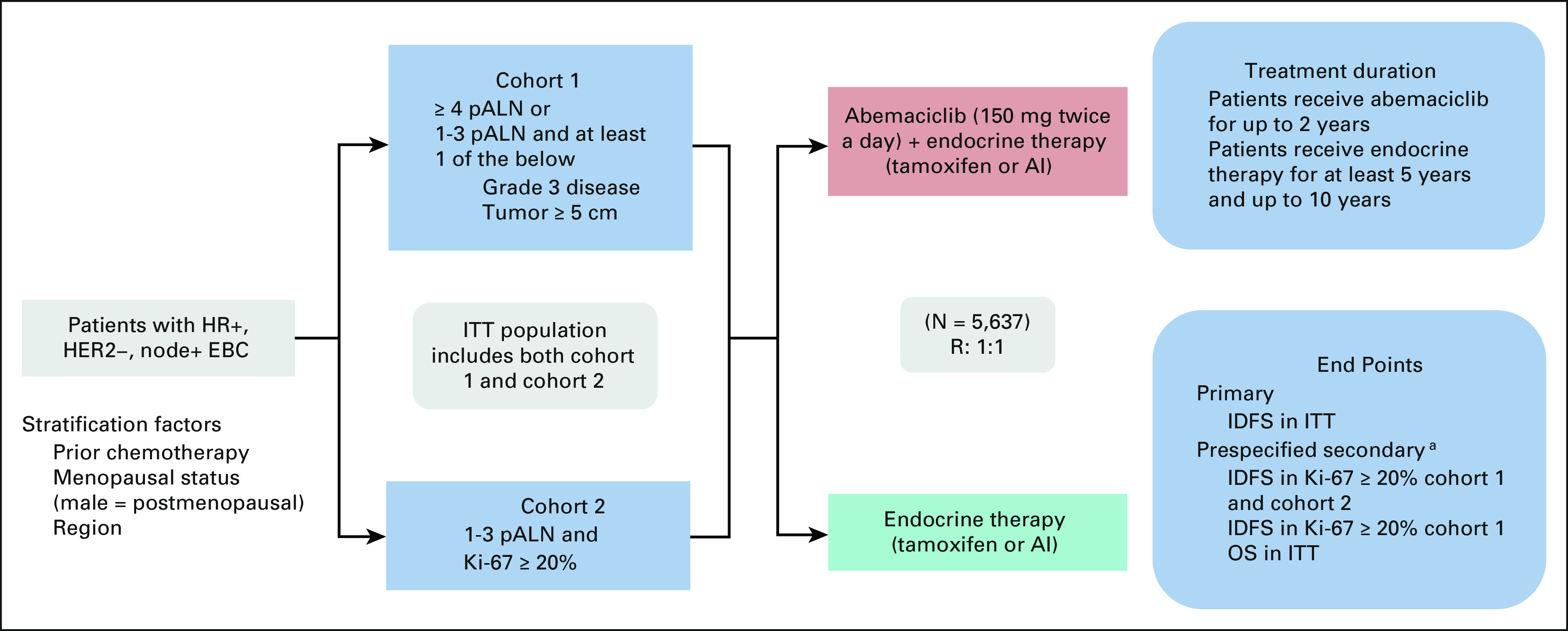
Trial schema of monarchE. Study design of monarchE, including key eligibility criteria and stratification factors, two enrollment cohorts, treatments arms (A = experimental—ET with abemaciclib v B = control—ET alone), treatment duration, and study end points. aA gated hierarchical testing strategy to control for type 1 error included three additional end points, IDFS in patients with Ki-67 score ≥ 20% from cohorts 1 and 2, followed by IDFS in patients with a Ki-67 score ≥ 20% from cohort 1 alone, and finally OS in the ITT population. AI, aromatase inhibitor; EBC, early breast cancer; ET, endocrine therapy; HER2–, human epidermal growth factor receptor 2–negative; HR+, hormone receptor–positive; IDFS, invasive disease-free survival; ITT, intent-to-treat; OS, overall survival; pALN, pathologic positive axillary lymph nodes; R, random assignment.
