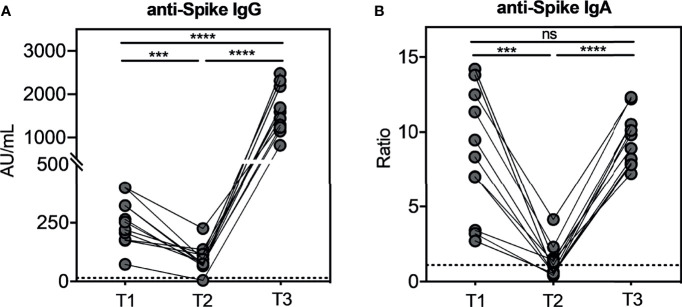
Figure 1.
Kinetic of total anti-SARS-CoV-2 IgG and IgA serum antibodies levels in seronegative recipients (ICs n=11) of Pfizer-BioNTech BNT162b2 mRNA-based vaccination. The evaluation of both serum antibodies was conducted at three weeks (T1) and nine months (T2) after the second dose, and three weeks after the booster dose (T3). In Figure (A) anti-SARS-CoV-2 S1/S2 IgG levels and in (B) anti-SARS-CoV-2 S1 IgA levels. The dotted lines correspond to IgG (> 15.0 AU/mL) and IgA (>1.1 Ratio) cut-off, respectively. The significance was determined using Tukey’s multiple comparisons test. One-way ANOVA, ***p=0.0002; ****p<0.0001, ns, not significant.