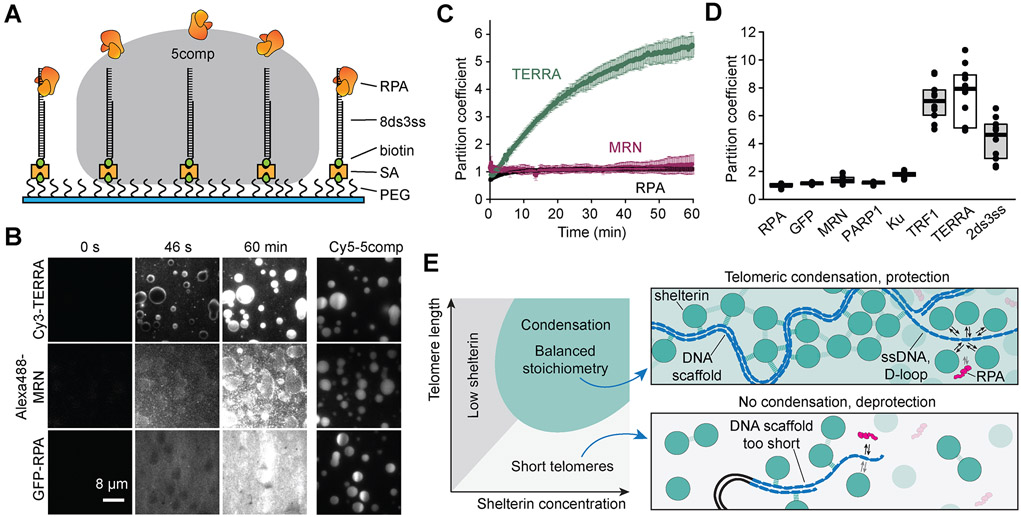
Figure 7. Shelterin droplets selectively recruit telomere-associated factors.
A. 5comp droplets are settled onto PEG surfaces decorated with 8ds3ss. (PEG: polyethylene glycol, SA: streptavidin; not to scale). B. 100 nM Cy3-TERRA, 15 nM Alexa488-MRN complex, or 100 nM GFP-RPA are introduced to 7.6 μM Cy5-5comp droplets. TERRA partitions strongly into the droplets, while MRN and GFP-RPA are initially excluded from the droplets and uniformly distributed after 60 min incubation. C. Partitioning of 100 nM Cy3-TERRA, 15 nM Alexa488-MRN, or 100 nM GFP-RPA into 7.6 μM 5comp droplets over time (mean ± SD, n = 3 droplets per condition). D. Partition coefficients of DDR proteins and telomere-associated factors in 7.6 μM 5comp droplets after 60 min incubation. The center and edges of the box represent the median with the first and third quartile (n = 10 droplets per condition). E. (Left) Multicomponent phase diagram of telomere condensation with balanced stoichiometry. No condensation results at low shelterin concentrations and/or short telomeres. (Top) Telomere condensation, formed by both heterotypic (dark dashes) and homotypic (light dashes) interactions, selectively recruit telomere-associated factors while acting as a diffusion barrier against other components that target telomeric DNA, like RPA. The enrichment of shelterin, and thus POT1, outcompetes RPA binding to ssTEL. (Bottom) Shortened telomere scaffold cannot recruit enough shelterin to form a condensate, which could fail to protect the ssTEL overhang against RPA binding. See also Figure S7 and Movie S5.