Figure 4. Prophylatic and cotreatment administration of C6G25S in treatment of SARS‐CoV‐2 and Delta variant in vivo .
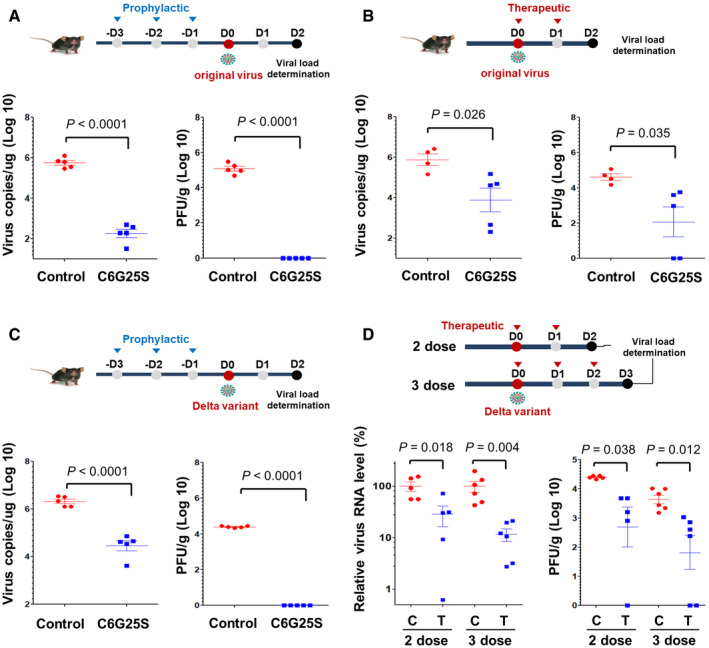
- K18‐hACE2 transgenic mice (Winkler et al, 2020) were treated once daily for 3 days before intranasal challenge with 104 plaque‐forming units (PFU) of the original virus. Prophylactic treatment consists of 30 min of AI (1.48 mg/l of C6G25S), followed by IN of 50 μg C6G25S. Mice receiving vehicle control (saline) for both AI and IN are annotated as control. Viral RNA (left) and infectious virions (right) in lungs were quantitated with RT‐qPCR and plaque forming assay, respectively, at 2 days postinfection (dpi).
- Mice were challenged intranasally with 104 PFU of virus and cotreatment with 1.48 mg/l of C6G25S or vehicle control (saline) by AI for 30 min on day 0 (right after infection) and day 1. Viral RNA and infectious virions were quantitated at 2 dpi.
- Prophylactic treatment against Delta virus with the same experimental design as in (A). Viral RNA (left) and infectious virions (right) in lungs were quantitated at 2 dpi.
- Cotreatment of C6G25S against Delta virus. The two‐dose group was treated at day 0 and day 1, and analyzed at day 2 dpi. The three‐dose group was treated at day 0, day 1, and day 2, and then analyzed at day 3 dpi. Virus RNA level was assessed relative to controls of each time point. The treatment group is labeled as T and the vehicle control is labeled as C.
Data information: Data are presented as mean ± SD. P‐value by Student’s t‐test.
Source data are available online for this figure.
