Figure EV4. CRISPR/Cas‐mediated gene repair.
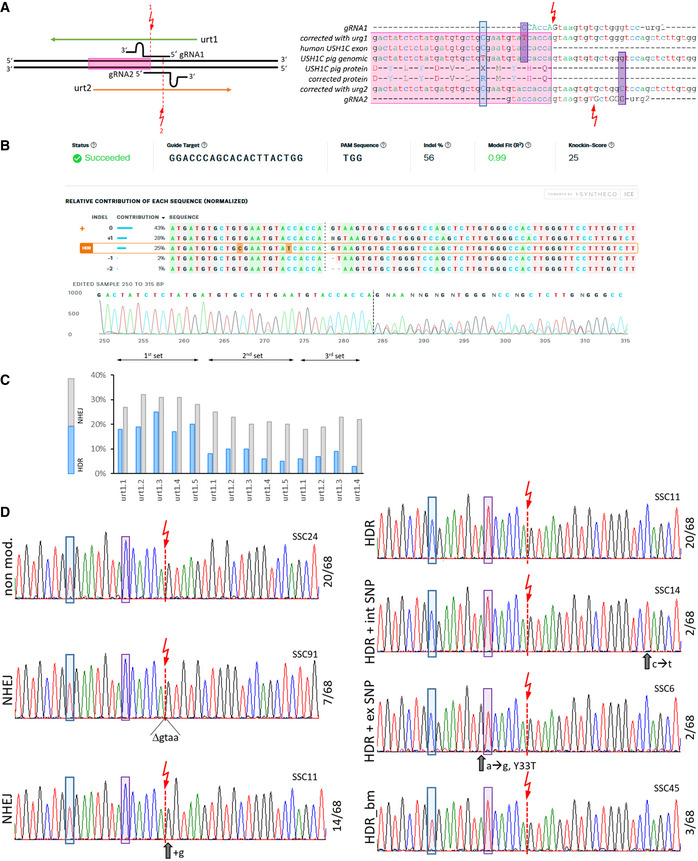
- In an initial approach the 2 oppositely oriented gRNA1 and gRNA2 and repair oligonucleotides urt1 and urt2 were tested for their efficacy to introduce NHEJ‐mediated indel formation. Exon 2 is marked by a pink box. The cutting sites of the Cas9 are shown as red arrows and the distinct positions at which the respective oligo‐nucleotides should introduce blocking mutations are indicated by magenta boxes. The position of T91 and its corrected variant C91 is indicated by a blue box.
- Sanger sequencing electropherograms were used to estimate efficacy of HDR after co‐transfection of plasmids expressing Cas9 and a gRNA and commercially synthesized ssODN repair templates into primary cells from USH1C pigs. PCR products from mixed cell clones were analyzed for NHEJ and HDR by the ICE CRISPR Analysis Tool.
- Optimization was performed with gRNA urt1 and five distinct repair oligo‐nucleotides in three independent experiments. The rate of HDR and NHEJ was determined as in (B).
- Single cells clones were generated from the pool nucleofected with gRNA1 and urt1.3 and analyzed by Sanger sequencing of PCR products. A diverse pattern of distinct modifications was observed in 68 examined single cell clones (SSCs). Representative electropherograms from designated SSCs are shown with the frequency of the respective pattern indicated at the right side. The cutting site of Cas9 as well as the correcting and blocking mutation are indicated as in (A). “non mod.” indicates SSCs without changes at the target site. NHEJ appeared either as deletions or insertions. HDR events were mostly restricted to the correct transformation of the correcting and blocking mutations. Some SSCs, however, showed accompanying mutations in the intronic (int SNP) or exonic (ex SNP) regions, with the latter potentially causing amino acid exchanges. Occasionally as well, HDR occurred only at the blocking mutation site, which is located closer to the cutting site than the correcting mutation site.
