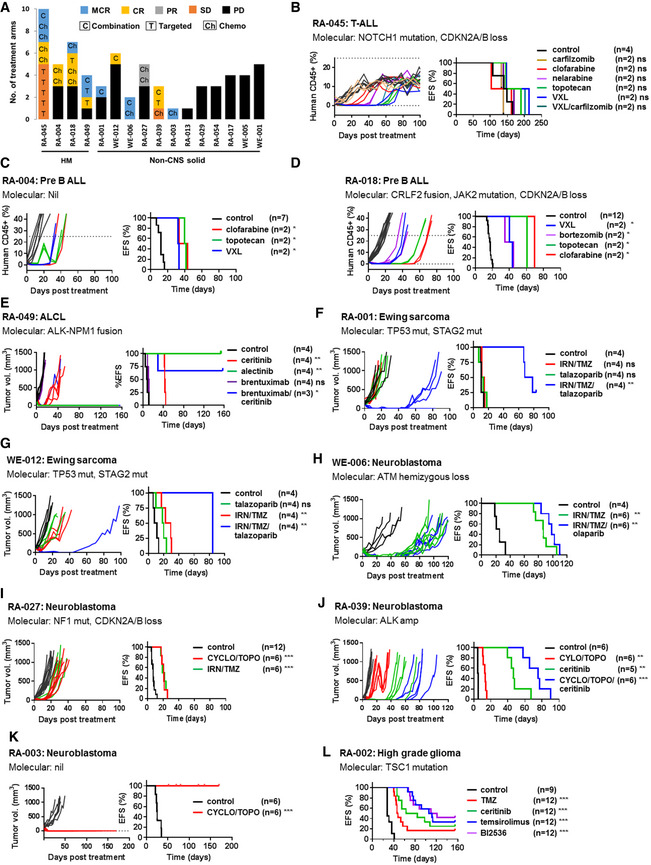
Figure 3. In vivo drug efficacy studies in patient‐derived xenografts.
-
ATreatment response in 16 hematologic malignancy (HM) and non‐CNS (central nervous system) solid patient‐derived xenograft (PDX) models. Objective responses including maintained complete response (MCR), complete response (CR), and partial response (PR) were observed in 10 of 16 models. Drugs are indicated as chemotherapy (Ch), targeted agent (T), or combination treatment (C).
-
B–DEvent‐free survival (EFS) and percentage of human CD45+ leukocytes in peripheral blood in three acute lymphoblastic leukemia (ALL) orthotopic models. An event is defined as human CD45 cells above 25% in the peripheral and is represented by the dotted line.
-
E–KEFS and tumor volume in seven non‐CNS subcutaneous PDX models which demonstrated objective response in one or more treatments.
-
LEFS in a CNS orthotopic model in which drug sensitivity was observed. EFS is time of inoculation of tumor cells to event (defined by neurologic symptoms or weight loss).
Data information: Survival curves were estimated for each treatment group using the Kaplan–Meier method and compared with the untreated control group in each PDX model statistically using log rank test. P value for log rank test for comparison of EFS: ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. The exact P values are provided in Appendix Table S6. ALCL, anaplastic large cell lymphoma; Cyclo, cyclophosphamide; IRN, irinotecan; PD, progressive disease; SD, stable disease; Topo, topotecan; TMZ, temozolomide; VXL, vincristine/dexamethasone/L‐asparaginase.
Source data are available online for this figure.
