Figure 1. FMRP is a novel IRE1 kinase substrate.
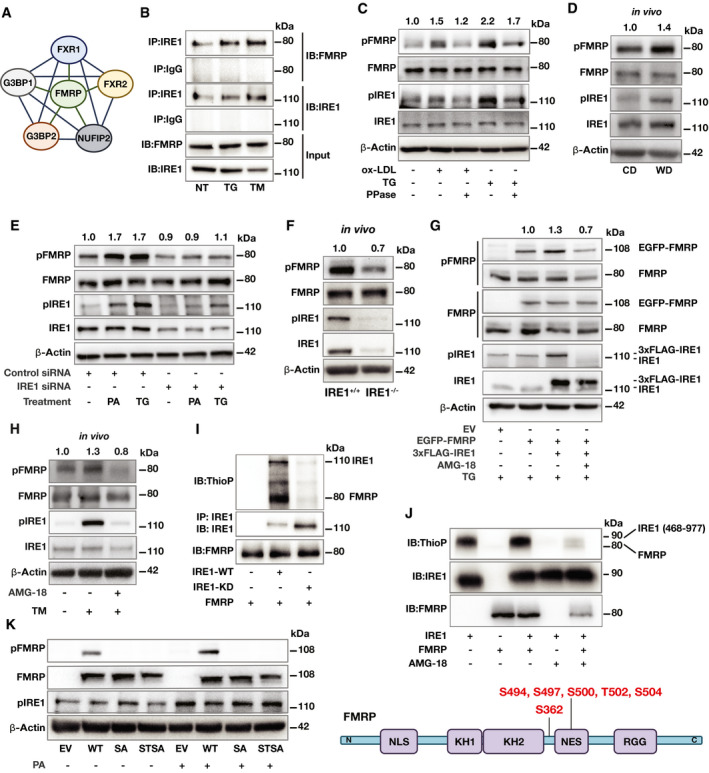
- STING analysis of published IRE1 interactome proteins in relation to FMRP (Acosta‐Alvear et al, 2018).
- HEK293T cells were co‐transfected with IRE1 and FMRP plasmids and stimulated with TG (600 nM) or TM (1 mg/ml) for 2 h. Protein lysates were immunoprecipitated (IP) with anti‐IRE1 or IgG (control) antibodies and analyzed by Western blotting using specific antibodies for FMRP and IRE1 (n = 3 biological replicates).
- RAW 264.7 mouse macrophages were treated with either oxLDL (50 µg/ml) or TG (300 nM) for 6 h. Protein lysates were treated with λ Phosphatase (PPase) for 30 min and analyzed by Western blotting using specific antibodies for pFMRP, FMRP, pIRE1, IRE1, and β‐Actin. pFMRP/FMRP fold induction is depicted above the blots (n = 6 biological replicates).
- Apoe−/− mice were fed with chow diet (CD) or western diet (WD) for 16 weeks followed by peritoneal macrophage (PM) isolation. Protein lysates were analyzed by Western blotting using specific antibodies for pFMRP, FMRP, pIRE1, IRE1, and β‐Actin. pFMRP/FMRP‐fold induction is depicted above the blots (n = 5 mice per group).
- Control‐ or IRE1‐siRNA transfected HEK293T cells were stimulated by either PA (500 µM) or TG (600 nM) for 4 h. Protein lysates were analyzed by Western blotting using specific antibodies for pFMRP, FMRP, pIRE1, IRE1, and β‐Actin. pFMRP/FMRP fold induction is depicted above the blots (n = 4 biological replicates).
- Protein lysates of thioglycolate‐elicited PM from IRE1α+/+ and IRE1α−/− mice (after 16 weeks on WD) were analyzed by Western blotting using specific antibodies for pFMRP, FMRP, pIRE1, IRE1, and β‐Actin. pFMRP/FMRP fold induction is depicted above the blots (n = 4 mice per group).
- MEF cells were transfected with either empty vector, EGFP‐FMRP or 3xFLAG‐IRE1 plasmids then pre‐treated either with vehicle (dimethyl sulfoxide, DMSO) or AMG‐18 (25 µM; 1 h) followed by TG (600 nM) stimulation for 4 h. Protein lysates were analyzed by Western blotting using specific antibodies for pFMRP, FMRP, pIRE1, IRE1, and β‐Actin. pFMRP/FMRP fold induction is depicted above the blots (n = 4 biological replicates).
- C57BL/6 were injected either with DMSO or AMG‐18 (30 mg/kg; 8 h), followed by TM injection (1 mg/kg; 8 h). Protein lysates of thioglycolate‐elicited PM were analyzed by Western blotting using antibodies for pFMRP, FMRP, pIRE1, IRE1, and β‐Actin. pFMRP/FMRP fold induction is depicted above the blots (n = 4 mice per group).
- HEK293T cells were transfected with either empty vector (EV), IRE1‐WT, or IRE1–KD plasmids and stimulated by TG (600 nM; 1 h). Protein lysates from each transfection were separately immunoprecipitated (IP) with anti‐IRE1 antibody and subjected to a kinase reaction with purified hFMRP protein and ATP‐γ‐S (100 µM) in kinase buffer. The IP protein were analyzed by Western blotting using specific antibodies for thiophosphate esters (ThioP), IRE1, and FMRP (n = 3 biological replicates).
- Purified FMRP and IRE1 kinase (activated) proteins were subjected to kinase assay and analyzed by Western blotting using specific antibodies for ThioP, IRE1, and FMRP (n = 3 biological replicates) and with LC‐MS/MS. Identified IRE1 kinase‐mediated FMRP phosphorylation sites (bottom).
- Fmr1−/− mouse embryonic fibroblasts (MEF) were transfected either with EV, WT‐FMRP, SA‐FMRP, or STSA‐FMRP plasmids followed by PA treatment (500 µM; 6 h). Protein lysates were analyzed by Western blotting using specific antibodies for FMRP, pFMRP, pIRE1, and β‐Actin (n = 3 biological replicates).
Data information: A representative blot is shown. In D, E, G, and H data are cumulative results of two independent experiments. Data are mean ± SEM. Unpaired t‐test with Welch’s correction or paired t‐test.
Source data are available online for this figure.
