Abstract
Background:
To illustrate challenges of imaging interpretation in patients with oligodendroglioma seen at a referral center and evaluate interrater reliability.
Methods:
Two neuro-oncologists reviewed diagnostic preradiation MRIs of oligodendroglioma patients; interrater reliability was calculated with the kappa coefficient (k). A neuroradiologist measured presurgical apparent diffusion coefficient (ADC), if available.
Results:
Extensive enhancement was noted in four of 58 patients, k = 0.7; necrosis in seven of 58, k = 0.61; calcification in seven of 17, k = 1.0; diffusion restriction in two of 39 patients, k = 1.0 (all only in grade 3). ADC values with receiver operator characteristic analysis for area under the curve were 0.473, not significantly different from the null hypothesis (p = 0.14).
Conclusions:
Extensive enhancement, necrosis and calcification correlated with grade 3 oligodendroglioma in our sample. However, interrater variability is an important limitation when assessing radiographic features, supporting the need for standardization of imaging protocols and their interpretation.
Keywords: : calcification, contrast enhancement, examiner concordance, interrater reliability, necrosis, oligodendroglioma, restricted diffusion
Oligodendrogliomas are diffusely infiltrative primary CNS tumors representing less than 10% of all gliomas [1]. A multidimensional approach is required to address challenges in oligodendroglioma and other primary rare CNS tumors [2]. Addressing these challenges is the aim of the NCI-CONNECT program at the National Cancer Institute (NCI) (https://ccr.cancer.gov/neuro-oncology-branch/connect). The WHO defines oligodendrogliomas molecularly by the presence of IDH mutation and codeletion of the short arm of chromosome 1 (1p) and the long arm of chromosome 19 (19q) [3,4]. Despite ongoing efforts to identify molecular alterations determining prognosis within molecularly defined oligodendrogliomas, little is known about optimal biomarkers for stratifying risk [5,6], and oligodendrogliomas are still classified on the basis of classical histological features into two grades, WHO grades 2 and 3 [7,8]. Tumor grading therefore has prognostic implications. Additionally, histological grading still drives therapeutic decisions in grade 2 gliomas, particularly oligodendrogliomas. For example, a ‘wait-and-see’ approach is often considered in patients with grade 2 gliomas in circumstances such as tumors located in unresectable locations or after gross total resection of a WHO grade 2 oligodendroglioma because delaying radiation therapy has not been shown to carry an adverse impact on overall survival [9,10]. However, such an approach is not common practice for grade 3 oligodendrogliomas.
After clinical presentation, imaging studies serve as the initial tool in the diagnosis of all brain tumors, including oligodendrogliomas. Tumor heterogeneity and location in the eloquent brain may hamper accurate diagnosis after a biopsy or incomplete resection. Previous studies have looked retrospectively into imaging features to predict tumor grade in oligodendrogliomas, using calcification, contrast enhancement [11], perfusion, and restricted diffusion [12]. However, there is no definitive set of imaging features to predict oligodendroglioma grading [13], likely in part because of the challenges in correctly identifying these features and variability among readers.
In this report, we retrospectively reviewed a cohort of patients with oligodendroglioma and attempted to validate imaging findings evaluated in previous studies as predictors of grading and evaluated inter-rater reliability.
Methods
All 58 evaluable patients had a centrally confirmed histopathological diagnosis of oligodendroglioma (molecularly confirmed: 54; not otherwise specified [NOS]: four). Appropriate imaging was available for review, performed preoperatively and/or before starting radiation therapy. MRI minimal sequences required were T1, T2, T2-FLAIR and T1 post-gadolinium contrast injection. The presence of calcification was only evaluated if CT scans were available (n = 17). Two neuro-oncologists (M Penas-Prado and MR Gilbert) with extensive clinical experience in the field of neurology and neuro-oncology, both blinded to histopathological grade and the radiology report, reviewed independently MRIs from all 58 patients. Reader interpretations for the following conventional MRI features were recorded: contrast enhancement (not present/partial/extensive), necrosis (yes/no), calcification (yes/no) and restricted diffusion (yes/no). ‘Partial’ contrast enhancement was defined as less than 50% of the 2D lesion area on axial imaging containing the largest area of contrast enhancement, and ‘extensive’ contrast enhancement was defined as ≥50% of the 2D lesion area showing contrast enhancement. T1 and T1 postcontrast images were evaluated when the presence of acute hemorrhage was suspected as a confounding factor for enhancement. ‘Necrosis’ was defined as the presence of a ring enhancing lesion on T1 postcontrast along with central hypointensity. The interrater reliability among the two neuro-oncologists was measured with Cohen’s kappa statistic. A neuroradiologist (R Shah), blinded to grade, measured presurgical apparent diffusion coefficient (ADC) when available (n = 21). Images were reviewed using Carestream Vue PACS Version 12.2.2.1025.
Results
Patients
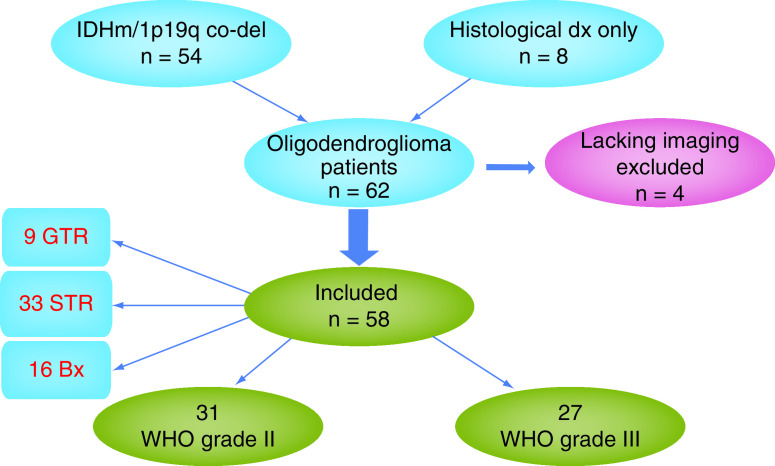
Thirty-one patients with oligodendroglioma WHO grade 2 and 31 patients with anaplastic oligodendroglioma WHO grade 3 were identified in the Neuro-Oncology Branch Natural History Study. As shown in Table 1, almost half of the patients presented with tumor in the frontal lobe. Fifty-four of the 62 patients had histopathological findings suggestive for oligodendroglioma along with IDH mutation either via immunohistochemistry staining or gene mutation testing, and 1p 19q codeletion by FISH testing performed on paraffin embedded tissue using a dual color probe set (positive deletion of the short arm of chromosome 1 including 1p36 and positive deletion of the long arm of chromosome 19 including 19q13). Four patients had a centrally confirmed morphological diagnosis of oligodendroglioma but either no IDH or no 1p19q testing; three did not have IDH testing, and one had no 1p19q status available but were also included in the analysis. We included these cases to avoid selection bias based on date of surgery (before required molecular diagnosis of oligodendroglioma). We excluded four patients (WHO grade 3) due to lack of imaging studies before receiving radiation to avoid misinterpretation of radiation-related imaging changes [14]. The total number of patients considered evaluable for analysis was 58. As detailed in Figure 1, among these 58 evaluable patients, 31 had grade 2 and 27 had grade 3 tumors; gross total resection was achieved in nine patients, subtotal resection in 33 patients and biopsy in 16.
Table 1. . Number of patients with specific tumor location.
| Frontal | Temporal | Parietal | Occipital | Multilobe | |
|---|---|---|---|---|---|
| Patient n | 32 | 14 | 4 | 1 | 11 |
Figure 1. . Diagram showing patients’ histology and extent of surgery.
Bx: Biopsy; GTR: Gross total resection; IDHm: IDH mutant: STR: Subtotal resection.
Contrast enhancement, necrosis & calcification
Two neuro-oncologists reviewed images from 58 evaluable patients. The first neuro-oncologist reported partial contrast enhancement (less than 50% of lesion area) or extensive contrast enhancement (more than 50% of lesion area) in 18 patients (six with WHO grade 2, 33%; 12 with WHO grade 3, 66%). The second neuro-oncologist reported partial or extensive contrast enhancement in 35 patients (15 with WHO grade 2, 43%; 20 with WHO grade 3, 57%); extensive enhancement was reported only in four and seven patients (all WHO grade 3) by readers 1 and 2, respectively.
Preradiation images from seven of 58 patients were reported to have necrosis (all WHO grade 3) as determined by the first neuro-oncologist and 11 patients (nine WHO grade 3) by the second neuro-oncologist. Calcification was noted in seven patients (all WHO grade 3) from the 17 patients with available CT head (nine WHO grade 3 and eight WHO grade 2) with both readers reporting same number. Examples shown in Figures 2 & 3.
Figure 2. . Representative images from a patient with oligodendroglioma WHO grade 2 (A–D).
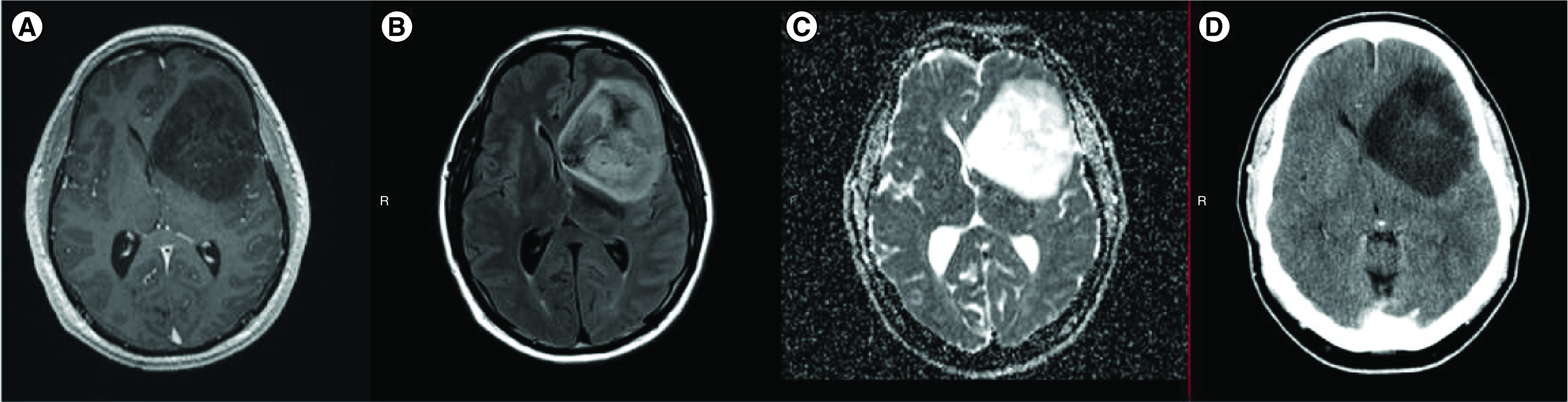
MRI axial T1 with contrast, axial fluid-attenuated inversion recovery (FLAIR), and axial apparent diffusion coefficient (ADC) ([A], [B] and [C], respectively), showing a large left frontal mass with no obvious contrast enhancement, FLAIR hyperintensity and high ADC values. (D) Axial CT head without contrast showing left frontal hypodensity without definitive calcification.
Figure 3. . Representative images from a patient with oligodendroglioma WHO grade 3 (A–D).
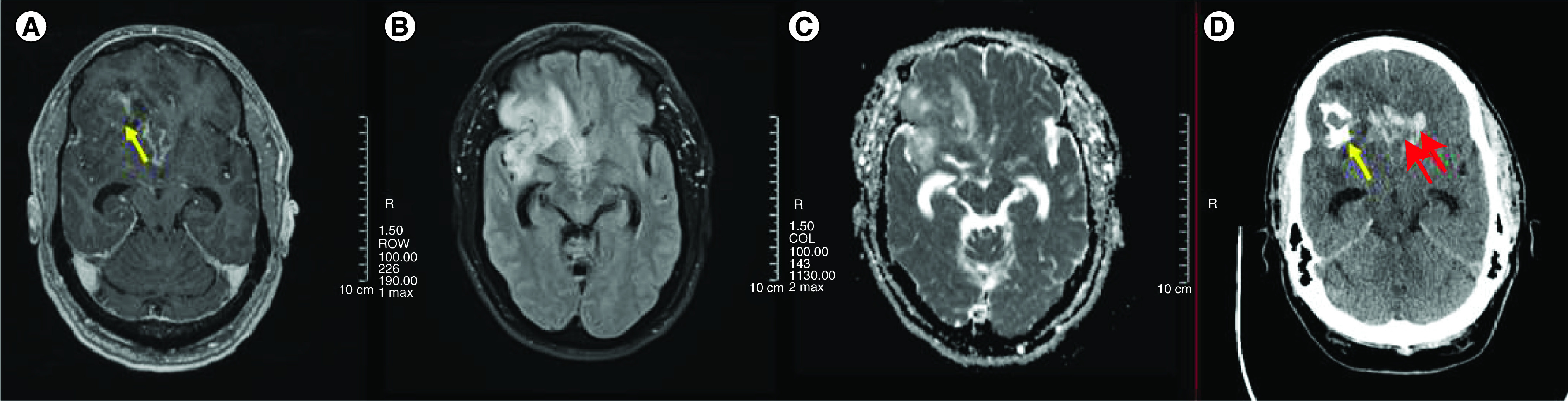
MRI Axial T1 with contrast, axial fluid-attenuated inversion recovery (FLAIR), and axial apparent diffusion coefficient (ADC) ([A], [B] and [C], respectively) showing right frontotemporal mass with contrast enhancement, FLAIR hyperintensity, and mixture of low and high ADC values. (D) Axial CT scan head without contrast showing right frontal calcification (single arrow) and bi-frontal intracranial hemorrhage (double arrow).
Cohen’s kappa statistic showed the following interrater agreement between the two neuro-oncologists in the preceding measures: interrater agreement on the contrast enhancement was k = 0.37 on grade 2 and k = 0.5 on grade 3; interrater agreement on extensive enhancement was k = 0.7. The agreement on necrosis interpretation was k = 0.61. There was complete agreement on the interpretation of calcification with k = 1.0. As shown in Table 2.
Table 2. . Interrater agreement: Cohen's kappa interrater agreement was used to assess agreement between readers, ranging from 0 (agreement by chance) to 1 (perfect agreement).
| All grades K (95% CI) |
Grade II K (95% CI) |
Grade III K (95% CI) |
|
|---|---|---|---|
| Contrast enhancement | k = 0.46 (0.27, 0.63) | k = 0.37 (0.12, 0.62) | k = 0.5 (0.23, 0.78) |
| Necrosis | k = 0.61 (0.33, 0.89) | k = unobtainable | k = 0.61 (0.34, 0.96) |
| Calcification | k = 1.0 | k = unobtainable | k = 1.0 |
| Diffusion-weighted imaging | k = 1.0 | k = unobtainable | k = 1.0 |
Apparent diffusion coefficient
Both neuro-oncologists reported restricted diffusion in only two of the 21 patients with available diffusion-weighted imaging (DWI) and ADC sequences, and both patients had WHO grade 3 tumors. Interrater agreement on restricted diffusion was k = 1.0. When calculating the ADC values with receiver operating characteristic analysis by a neuroradiologist, the area under the curve was 0.473, which was not significantly different from 0.5 (null hypothesis; p = 0.14).
Discussion
Patients with suspected brain tumors discovered on imaging are usually referred for surgical intervention because this provides tumor tissue for histological diagnosis and molecular testing and the extent of resection is an important prognostic factor. Although MRI is the best noninvasive technique to diagnose brain tumors, there is no set of imaging features that fully predicts tumor histology or histological grade with a high level of accuracy, and interpretation of these features is not easy to standardize. This is particularly relevant in the context of deep tumors or those in eloquent brain where extensive (or even subtotal) resection may not be possible. In these situations, it would be useful if conventional imaging findings (contrast enhancement, necrosis, calcification) helped predict the most malignant component of the tumor to guide a biopsy, thereby reducing the likelihood of ‘undergrading’ the tumor.
Contrast enhancement is reported in 40–60% of oligodendrogliomas in different studies, and enhancement has been suggested as an imaging feature indicating higher grade [15,16]. However, the ‘chicken-wire’ capillary network is believed to be behind the contrast enhancement occasionally observed in low-grade tumors [17]. In our study, contrast enhancement (of any amount) was noted in 31 and 60% of all cases by the readers, respectively; extensive contrast enhancement (more than 50% of the lesion area) was noted only in grade 3 tumors by both readers (in 15 and 26%, respectively). Thus, the presence of an extensive area of contrast enhancement can be helpful in predicting higher grade, but not all high-grade oligodendrogliomas show contrast enhancement. The origin of contrast enhancement is thought to be related to neovascularization of the tumor [18]. However, this needs to be interpreted with caution as previous studies showed that contrast enhancement is not a specific feature to simply differentiate grade 2 from grade 3 oligodendroglioma [19].
Necrosis was noted by the first reader in 12% of our sample patients (all of them were grade 3 in histology) and reported by the second reader in 19% (82% of those were grade 3), suggesting this feature increases diagnostic confidence to predict a higher-grade pathology. Of note, the presence of necrosis in oligodendroglioma does not carry the same unfavorable implication as in astrocytic tumors. In a study published in 2006, necrosis was a predictor for poor overall survival in anaplastic oligoastrocytoma but not in anaplastic oligodendrogliomas [20].
In our small sample of patients (only 17 had pre-surgical CT head available for review), calcification was noted by both readers only in grade 3 tumors (seven patients had calcification out of nine WHO grade 3 with available pre-surgical CT head). Although a larger sample size would be needed to reach stronger conclusions, the high frequency of calcification is of interest. Calcification is commonly reported in oligodendroglioma and could suggest 1p/19q co-deleted tumor [21]. In concordance with our data, a previous study suggested that calcification is more common in grade 3 oligodendroglioma, although this was not statistically significant [12].
ADC sequence depends on the diffusion of water molecules within the tissue and correlates with the cellularity of such tissue. This sequence was proposed to ‘predict’ the glioma grade and help identify appropriate tumor biopsy sites [22]. In agreement with previous observations suggesting that diffusion restriction is not commonly reported in oligodendroglioma, we noticed restricted diffusion in only 9.5% of our samples (two of 21 patients), and both patients had anaplastic oligodendroglioma [23] Khalid et al. [12] proposed a ADC cutoff below which higher grade can be predicted. However, when calculating the ADC values with ROC analysis for our sample the AUC did not meet predetermined significance cutoff, implying the ADC value may not be a reliable indicator of a grade 3 tumor.
Notwithstanding the potential role of conventional MRI features in noninvasively predicting grading, in this article, we call attention to the pitfalls of identifying these features by reporting the interrater agreement between two expert neurologists/neuro-oncologists when interpreting MRI scans of patients with WHO grade 2 and 3 oligodendroglioma. Our goal was to recapitulate common issues found in clinical practice, especially in clinical settings where there is no immediate access to neuroradiologists with expertise in primary brain tumors, or in referral centers where patients request a new opinion providing previous imaging studies obtained at several institutions. Patients are commonly referred for consultation from multiple other academic and/or private practices with variable experience in the imaging diagnosis and management of primary brain tumors. Notably, interrater agreement was poor for interpretation of contrast enhancement in grade 2 tumors. Upon additional review, common reasons for disagreement were the presence of small areas of enhancement that were considered normal vasculature and variations in the amount of enhancement due to heterogeneous techniques used in studies obtained at different institutions. Conversely, there was good interrater agreement in interpreting the following measures: presence of any amount of contrast enhancement in grade 3 oligodendroglioma, extensive contrast enhancement and necrosis. Complete agreement was reported in interpretation of calcification and restricted diffusion, although the number of appropriate studies to evaluate these features was smaller. Our observations, when taken together with findings from previous studies in which experts were shown to have higher agreement than novices in imaging interpretation [24], highlight the limitations of imaging interpretation and the need to use a homogenous MRI protocol across different centers to decrease variability in interpretation due to technical factors. Importantly, a Brain Tumor Imaging Protocol for standardization of imaging in neuro-oncology has already been proposed [25], and even though it is primarily designed for incorporation of imaging in multicenter clinical trials, our findings support the need to incorporate this standard into routine clinical imaging to decrease variability of imaging interpretation.
Our study had several limitations. Sample size was relatively small, although comparable with a previous study reporting similar findings in 75 patients [12]. Because this was a retrospective study, there were limitations regarding the availability of imaging studies (i.e., CTs) before surgical intervention, or the availability of specific sequences (DWI, ADC); however, this reflects the real-world challenges of imaging interpretation, especially by clinicians in their clinical practice, needing to evaluate and compare imaging studies obtained with variable protocols. Moreover, ADC values could vary depending on multiple factors (different scanners, software, etc.), and this might be the reason why the use of ADC is limited in clinical practice [26]. Adding advanced imaging technology to conventional MRI is showing promise to help obtain better data for grading oligodendrogliomas, but further study is needed [27]. About one-quarter of the patients included in our series underwent biopsy only, which could have assigned an incorrect grading to the tumor due to sample error; hence our study goal of trying to decrease the chance of biopsy target error due to intratumoral heterogeneity [28]. Seven patients were originally diagnosed before the implementation of molecular requirements for diagnosis of oligodendroglioma by the WHO 2016 classification (IDH mutations and 1p19q codeletion), and whereas these tumors were centrally confirmed as histological oligodendrogliomas, they can only be classified as oligodendroglioma or anaplastic oligodendroglioma NOS based on current WHO guidelines. However, the inclusion of this small number of tumors without full molecular confirmation (which could potentially be molecular astrocytoma or other rare tumor entities) does not invalidate the analysis of imaging features for prediction of grade and the analysis of low interrater agreement when evaluating the same imaging studies because these challenges likely apply to imaging interpretation of all gliomas and brain tumors in general. Importantly, the relevance of histological grade to predict prognosis in oligodendroglioma is being revisited. Recent retrospective data have suggested superior importance of molecular profile over histological grading (2 vs 3) to predict prognosis of diffuse glioma patients in general [29] and oligodendroglioma in particular [30]. Although these studies convey valid points, they need confirmation in prospective studies, and histological grading is still included in the WHO 2016 and current WHO 2021 classification of gliomas and it is shown to affect survival independent from other factors in IDH mutant tumors [4,31,32]. Hence, histological grading continues to bear importance in clinical decision-making in oligodendrogliomas and other gliomas.
Finally, although the imaging technique was not homogenous across all studies, and we reported the inter-rater agreement between two non-radiologists, we consider these strengths of our study as they reflect the challenges of daily clinical practice and the importance of introduction of homogeneous imaging protocols for evaluation of brain tumors to minimize variability in interpretation. A prospective study using this homogeneous imaging protocol, introducing standard quantification of findings and evaluating interrater readings would help establish the value of this protocol in decreasing reading variability and predicting grade more accurately.
Conclusion
In our patient population with oligodendroglioma, preoperative brain imaging demonstrating extensive enhancement, necrosis and calcification suggested higher tumor grade. These findings may provide a guide for the optimal biopsy site if extensive resection is not feasible. However, imaging interpretation is variable among readers, underscoring the need for both standardized imaging protocols and reproducible quantification of findings such as contrast enhancement, calcifications and provision of measurements of calculated values such as ADC.
Summary points.
In keeping with previous studies, areas with extensive enhancement and/or necrosis were associated with higher grade.
Such areas with extensive enhancement and/or necrosis can guide biopsies if resection is not feasible.
Our findings of interobserver discrepancies call attention to the pitfalls of imaging interpretation in a real-world setting.
Our findings underscore the need for both standardized brain tumor imaging protocols to decrease variability of imaging acquisition at different institutions and quantification of findings, such as contrast enhancement and calcifications.
This study is the first to explore the difficulties of visual interpretation, even among expert clinicians in predicting oligodendroglioma grading.
Footnotes
Authorship
All listed authors participated in the writing of the manuscript and have read and approved the final version.
Financial & competing interests disclosure
The authors are members of The NCI Comprehensive Oncology Network for Evaluating Rare CNS Tumors (NCI-CONNECT), which is a program within the Rare Tumor Patient Engagement Network (RTPEN), an initiative supported by the Cancer Moonshot funds and managed at the National Institutes of Health, National Cancer Institute, Center for Cancer Research, Neuro-Oncology Branch. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Ostrom QT, Cioffi G, Gittleman H et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro. Oncol. 21(Suppl. 5), v1–v100 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Penas-Prado M, Wu J, Cahill DP et al. Proceedings of the Comprehensive Oncology Network Evaluating Rare CNS Tumors (NCI-CONNECT) Oligodendroglioma Workshop. Neuro-Oncol. Advances. 2(1), vdz048 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article discusses challenges in oligodendroglioma treatment and future directions.
- 3.Louis DN, Perry A, Reifenberger G et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta. Neuropathol. 131(6), 803–20 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Louis DN, Perry A, Wesseling P et al. The 2021 WHO Classification of Tumors of the Central Nervous System: a summary. Neuro. Oncol. 23(8), 1231–51 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This article is important in defining oligodenroglioma and differentiating it from other rare diseases.
- 5.Aoki K, Nakamura H, Suzuki H et al. Prognostic relevance of genetic alterations in diffuse lower-grade gliomas. Neuro. Oncol. 20(1), 66–77 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Halani SH, Yousefi S, Velazquez Vega J et al. Multi-faceted computational assessment of risk and progression in oligodendroglioma implicates NOTCH and PI3K pathways. NPJ Precis. Oncol. 2018. 2, 24 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahill DP, Louis DN, Cairncross JG. Molecular background of oligodendroglioma: 1p/19q, IDH, TERT, CIC and FUBP1. CNS Oncol. 2015. 4(5), 287–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bettegowda C, Agrawal N, Jiao Y et al. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 333(6048), 1453–5 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van den Bent MJ, Afra D, de Witte O et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 366(9490), 985–90 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Dhawan S, Patil CG, Chen C, Venteicher AS. Early versus delayed postoperative radiotherapy for treatment of low-grade gliomas. Cochrane Database Syst Rev. 2020. 1, CD009229 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonardi MA, Lumenta CB. Oligodendrogliomas in the CT/MR-era. Acta Neurochir. 143(12), 1195–203 (2001). [DOI] [PubMed] [Google Scholar]
- 12.Khalid L, Carone M, Dumrongpisutikul N et al. Imaging characteristics of oligodendrogliomas that predict grade. AJNR Am. J. Neuroradiol. 33(5), 852–7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• This article supports our findings.
- 13.Jr Alvord EC. Is necrosis helpful in the grading of gliomas? Editorial opinion. J. Neuropathol. Exp. Neurol. 51(2), 127–32 (1992). [DOI] [PubMed] [Google Scholar]
- 14.Walker AJ, Ruzevick J, Malayeri AA et al. Postradiation imaging changes in the CNS: how can we differentiate between treatment effect and disease progression? Future Oncol. 10(7), 1277–97 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fortin D, Cairncross GJ, Cairncross GJ. Oligodendroglioma: an appraisal of recent data pertaining to diagnosis and treatment. Neurosurg. 45(6), 1279–91 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Reiche W, Grunwald I, Hermann K et al. Oligodendrogliomas: a comparison of CT and MR imaging features with histological malignancy grading in 20 cases: A pathoradiological study. Acta Radiologica. 43(5), 474–82 (2002). [PubMed] [Google Scholar]
- 17.Smits M. Imaging of oligodendroglioma. Br. J. Radiol. 89(1060), 20150857 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daumas-Duport C, Tucker ML, Kolles H et al. Oligodendrogliomas. Part II: a new grading system based on morphological and imaging criteria. J. Neuro-oncol. 34(1), 61–78 (1997). [DOI] [PubMed] [Google Scholar]
- 19.White ML, Zhang Y, Kirby P, Ryken TC. Can tumor contrast enhancement be used as a criterion for differentiating tumor grades of oligodendrogliomas? AJNR Am. J. Neuroradiol. 26(4), 784–90 (2005). [PMC free article] [PubMed] [Google Scholar]
- 20.Miller CR, Dunham CP, Scheithauer BW, Perry A. Significance of necrosis in grading of oligodendroglial neoplasms: a clinicopathologic and genetic study of newly diagnosed high-grade gliomas. J. Clin. Oncol. 24(34), 5419–26 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Lasocki A, Gaillard F, Gorelik A, Gonzales M. MRI features can predict 1p/19q status in intracranial gliomas. AJNR Am. J. Neuroradiol. 39(4), 687–92 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darbar A, Waqas M, Enam SF, Mahmood SD. Use of preoperative apparent diffusion coefficients to predict brain tumor grade. Cureus. 10(3), e2284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koeller KK, Rushing EJ. From the archives of the AFIP: oligodendroglioma and its variants: radiologic-pathologic correlation. Radiographics. 25(6), 1669–88 (2005). [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Muller DMJ, van Duijn RJM et al. Inter-rater agreement in glioma segmentations on longitudinal MRI. NeuroImage. Clin. 22, 101727 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellingson BM, Bendszus M, Boxerman J et al. Consensus recommendations for a standardized Brain Tumor Imaging Protocol in clinical trials. Neuro. Oncol. 17(9), 1188–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huo J, Alger J, Kim H et al. Between-scanner and between-visit variation in normal white matter apparent diffusion coefficient values in the setting of a multi-center clinical trial. Clin. Neuroradiol. 26(4), 423–30 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Lin Y, Xing Z, She D et al. IDH mutant and 1p/19q co-deleted oligodendrogliomas: tumor grade stratification using diffusion-, susceptibility-, and perfusion-weighted MRI. Neuroradiology. 59(6), 555–62 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muragaki Y, Chernov M, Maruyama T et al. Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim. Invasive Neurosurg. 51(5), 275–9 (2008). [DOI] [PubMed] [Google Scholar]
- 29.Olar A, Wani KM, Alfaro-Munoz KD et al. IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol. 129(4), 585–96 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamoun A, Idbaih A, Dehais C et al. Integrated multi-omics analysis of oligodendroglial tumours identifies three subgroups of 1p/19q co-deleted gliomas. Nat Commun. 7, 11263 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller JJ, Loebel F, Juratli TA et al. Accelerated progression of IDH mutant glioma after first recurrence. Neuro Oncol. 21(5), 669–77 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Franceschi E, Tosoni A, Bartolini S et al. Histopathological grading affects survival in patients with IDH-mutant grade II and grade III diffuse gliomas. Eur. J. Cancer. 137, 10–7 (2020). [DOI] [PubMed] [Google Scholar]; •• This article discusses the impact of oligodedroglioma grading in prognosis.