Abstract
Introduction:
Pancreatic neuroendocrine tumor (PNET) survival outcomes differ by race. Current recommendations for surveillance of PNETs <2cm in size is based on low malignant potential and low rates of lymph node metastases (LNM). We investigated whether these guidelines are universally applicable, regardless of race.
Methods:
A multi-institutional analysis of patients with resected, nonfunctional, sporadic PNETs was performed initially using the US Neuroendocrine Study Group dataset with the National Cancer Database as a validation dataset. Patients with distant metastatic disease were excluded from analysis.
Results:
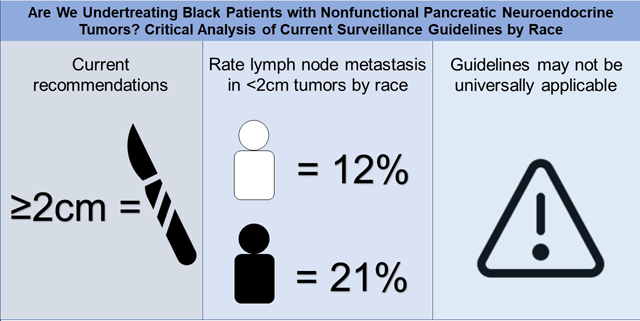
A total of 453 (388 White and 65 Black) and 5532 patients (4772 White and 760 Black) were analyzed in the initial and validation datasets, respectively. White patients had a low incidence of LNM in <2cm tumors in both datasets (5% and 12%, respectively), which increased with tumor size. However, the incidence of LNM in Black patients was similar in the initial and validation datasets for tumors sized <2cm (23% and 21%) and 2–3cm (21% and 29%). Black patients had a significantly higher incidence of LNM in <2cm tumors in both the initial and validation datasets (p<0.01) compared to White patients.
Conclusions:
The current recommendation for surveillance of <2cm PNETs is likely based on a low rate of LNM seen in a predominantly White population. The incidence of LNM in Black patients with <2cm tumors is clinically relevant and concerning. Current guidelines may not be universally applicable and a more aggressive approach towards resection in Black patients with small PNETs may be warranted.
Keywords: Pancreatic Neuroendocrine Tumor, Racial Disparity, Tumor Size
Precis
Black patients with pancreatic neuroendocrine tumors are more likely than White patients to have lymph node metastases with smaller tumors; current size-based guidelines are not likely applicable to this patient population.
Graphical Abstract
Introduction
Pancreatic neuroendocrine tumors (PNETs) are the second most common pancreatic malignancy and the incidence of disease has been rising.(1) These neoplasms can be classified as either functional or nonfunctional based on their ability to secrete bioactive peptides. These peptides can cause debilitating symptoms such as diarrhea, reflux, rash, altered glucose metabolism, or abdominal pain.(2) Nonfunctional PNETs are often found incidentally on cross sectional imaging or present with symptoms due to tumor mass effect, such as jaundice, nausea, vomiting, or weight loss.(3, 4) PNETs are a heterogeneous group of tumors with varying malignant potential, ranging from <10% for insulinomas to nearly 100% in gastrinomas.(3) Since the range of malignant potential is wide, it is important to find factors differentiating indolent tumors from aggressive cancers as systemic treatments are limited and often the only chance for cure is resection.(5)
Several PNET characteristics have been shown to correlate with unfavorable recurrence-free and overall survival; including tumor grade, tumor size, ki67% indices, microscopic invasion, lymph node involvement, and presence of metastatic disease.(6–8). A recent meta-analysis has shown that nonfunctional PNETs smaller than 2 cm have a 11% risk of LNM, while those larger than 2 cm have a nearly 40% risk of LNM at resection.(9) This lower risk of LNM in smaller tumors has been used to guide current clinical guideline recommendations regarding surgical resection for tumors over 2cm.(6, 10–15)
Previous studies have found differences in PNET presentation and outcomes between Black and White patients. Analyses of the SEER database showed that Black patients presented with more advanced disease with decreased overall survival without surgical resection. (1, 16) Interestingly, the United States Neuroendocrine Tumor Study Group (USNETG) previously showed that Black patients had a better 5-year disease free survival (DFS) for node-negative PNETs than White patients, while DFS was comparable between racial groups with node-positive disease.(17) We recently reported significant racial differences in PNET size on presentation and disease free recurrence in Black patients from our institutional dataset.(18) Given the racially disparate outcomes reported in PNETs and the significance tumor size plays on clinical decision making, we sought to determine if current clinical guidelines using tumor size as a surrogate for LNM were predictive in Black patients with PNETs.
Materials and Methods
Patient Selection
A retrospective analysis of patients with sporadic, nonfunctional pancreatic neuroendocrine tumors undergoing non-emergent, curative intent resection between the years 1998 and 2019 was performed. The initial dataset combined our institutional data with that from the USNETSG. The validation dataset was obtained from the National Cancer Database (NCDB) between 2004 and 2017. Patients without lymph node resections or with synchronous distant disease were excluded from both datasets. Grade 3 tumors were excluded from the USNETSG dataset. The NCDB does not include grade, but poorly differentiated or undifferentiated tumors were excluded. Malignancy was defined as having LNM on final pathology. Disease free survival (DFS) was defined as time from surgery to radiographic or biopsy-proven recurrence. Recurrence and granular details on operation type is not reported in the NCDB dataset.
Statistics
Fisher’s Exact and Chi-squared tests were used to compare categorical variables. Mann-Whitney Rank sum test was used to compare group medians. Survival was determined by Kaplan-Meier methodology and logistic regression analysis was utilized to determine the impact of clinicopathologic variables as pre-operative predictors of malignancy. MedCalc® Statistical Software version 20.019 (MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2021) was utilized for analyses. Statistical significance defined as P < 0.05.
Results
Patient Demographics and Tumor Characteristics
Within the initial dataset, a total of 453 patients underwent resection, 389 (86%) were White and 65 (14%) were Black. In the validation dataset, a total of 5532 patients had surgery, 4772 (86%) were White and 760 (14%) were Black. Patient and tumor characteristics for the initial and validation datasets were similar (Table 1). In both datasets, Black patients tended to be younger and more likely female than White patients. Black patients were more likely to have positive lymph nodes in both the initial (23% vs 21%; p=0.02) and the validation datasets (32% vs 27%; p=<0.01). Degree of differentiation was similar between racial cohorts in the initial dataset, but Black patients were more likely to have well differentiated tumors in the validation dataset (85% vs 80%; p=<0.01). The validation dataset does not report types of resection, recurrence, WHO grade, or length of follow-up.
Table 1.
Patient Characteristics
| Characteristic | Initial dataset | Validation dataset | ||||||
|---|---|---|---|---|---|---|---|---|
| All (n=453) | White patients (n=388) | Black patients (n=65) | p Value | All (n=5532) | White patients (n=4472) | Black patients (n=760) | p Value | |
| Age, y, median (IQR) | 61 (51 – 67) | 61 (52 – 68) | 58 (48 – 65) | 0.04 | 60 (50 – 68) | 60 (51 – 68) | 56 (48 – 64) | <0.01 |
| Age >65 y, n (%) | 162 (36) | 145 (37) | 18 (28) | 0.07 | 1966 (36) | 1791 (38) | 175 (23) | <0.01 |
| Sex, f, n (%) | 217 (48) | 177 (46) | 40 (61) | 0.02 | 2696 (49) | 2197 (46) | 499 (66) | <0.01 |
| Tumor size, cm, median (IQR) | 2.4 (1.5 – 3.8) | 2.4 (1.5 – 3.9) | 2.1 (1.4 – 3.1) | 0.11 | 2.5 (1.6 – 4.2) | 2.5 (1.6 – 4.2) | 2.5 (1.5 – 4.3) | 0.95 |
| Size >2cm, n (%) | 272 (60) | 237 (61) | 35 (54) | 0.052 | 3678 (66) | 3176 (67) | 502 (66) | 0.79 |
| Size category, n (%) | ||||||||
| <2 cm | 181 (40) | 151 (39) | 30 (46) | 0.28 | 1854 (34) | 1596 (33) | 258 (34) | 0.73 |
| 2–3 cm | 97 (21) | 84 (21) | 13 (20) | - | 1240 (22) | 1084 (23) | 156 (21) | - |
| >3 cm | 175 (39) | 153 (40) | 22 (34) | - | 2438 (44) | 2092 (44) | 346 (45) | - |
| Type of operation, n (%) | ||||||||
| Distal | 283 (62) | 245 (63) | 38 (58) | 0.61 | NA | NA | NA | - |
| Whipple | 148 (33) | 128 (33) | 20 (31) | - | NA | NA | NA | - |
| Undefined | 8 (2) | 5 (1) | 3 (5) | - | NA | NA | NA | - |
| Central | 12 (3) | 8 (2) | 4 (6) | - | NA | NA | NA | - |
| Total | 2 (0) | 2 (1) | 0 (0) | - | NA | NA | NA | - |
| R0, n (%) | 392 (87) | 333 (86) | 59 (91) | 0.32 | 4997 (95) | 4312 (95) | 685 (95) | 0.62 |
| No. lymph nodes resected, median (IQR) | 10 (16 – 23) | 10 (9 –11) | 10 (8 – 13) | 0.47 | 10 (10–10) | 10 (10–10) | 10 (9–11) | 0.97 |
| Node positive, n (%) | 97 (21) | 82 (21) | 15 (23) | 0.02 | 1524 (28) | 1282 (27) | 242 (32) | <0.01 |
| Differentiation, n (%) | ||||||||
| Well | 372 (87) | 317 (86) | 55 (89) | 0.62 | 4462 (81) | 3813 (80) | 649 (85) | <0.01 |
| Intermediate | 57 (13) | 50 (14) | 7 (11) | - | 1070 (19) | 959 (20) | 111 (15) | - |
| WHO Grade, n (%) | ||||||||
| 1 | 285 (63) | 240 (62) | 45 (69) | 0.26 | NA | NA | NA | - |
| 2 | 168 (37) | 148 (38) | 20 (31) | NA | NA | NA | - | |
| Recurred, n (%) | 51 (11) | 48 (12) | 3 (5) | 0.09 | NA | NA | NA | - |
| Follow-up, mo, median (IQR) | 32 (13 – 60) | 33 (13 – 61) | 31 (12 – 55) | 0.26 | NA | NA | NA | - |
NA, data not available for analysis
Pre-operative Predictors of LNM
Logistic regression analysis was performed to determine correlations between age >65 years, sex, and tumor size for both datasets (Table 2). Looking at all patients in the initial dataset, patient age >65 years at diagnosis was not a significant predictor of LNM, but it was in the validation dataset for White patients only (OR 0.9; p=0.04). Female sex was found to be protective of LNM in the initial dataset for White patients (OR 0.6; p=0.03), but was not in the validation dataset for any patient cohort. Tumor size as a continuous variable was found to be significant in both the initial (OR 1.18; p=<0.01) and validation datasets (OR 1.0011; p=<0.01) for White patients only. When tumor size is studied dichotomously using the clinically relevant size cut-off of 2 cm, tumors >2 cm had increased risk of LNM in both initial (OR 8.1; p=<0.01) and validation (OR 3.9; p=<0.01) datasets for White patients, but only in the validation dataset for Black patients (OR 2.3; p=<0.01). Looking more granularly at tumor sizes above 2 cm, we find that only tumor sizes greater than 3 cm have an increased risk for LNM in Black patients (OR 2.7; p=<0.01) and not tumors in the 2–3 cm range.
Table 2.
Logistic Regression Analysis of Preoperative Predictors of Malignancy
| Characteristic | All (n=453) | White patients (n=388) | Black patients (n=65) | |||
|---|---|---|---|---|---|---|
| OR [95% CI] | p Value | OR [95% CI] | p Value | OR [95% CI] | p Value | |
| Initial dataset | ||||||
| Age >65 y | 0.8 [0.5 – 1.3] | 0.38 | 0.8 [0.5 – 1.4] | 0.47 | 0.6 [0.1 – 2.4] | 0.44 |
| Female sex | 0.6 [0.4 – 0.9] | 0.02 | 0.6 [0.3 – 0.9] | 0.03 | 0.6 [0.2 – 2.1] | 0.46 |
| Size, cm | 1.15 [1.07 – 1.23] | <0.01 | 1.18 [1.09 –1.28] | <0.01 | 1.01 [0.81 – 1.25] | 0.92 |
| Size >2 cm | 5.2 [3.0 – 9.2] | < 0.0001 | 8.1 [3.8 – 17.4] | < 0.01 | 1.0 [ 0.3 – 3.1] | 0.96 |
| Size category | ||||||
| <2 cm | ref | ref | ref | |||
| 2–3 cm | 2.5 [1.2 – 5.2] | 0.01 | 3.9 [1.6 – 9.6] | 0.01 | 1.0 [0.2 – 4.6] | 0.99 |
| >3 cm | 6.9 [3.9 – 12.4] | <0.01 | 11.2 [5.1 – 24.5] | <0.01 | 1.0 [0.3 –3.6] | 0.96 |
| Validation dataset | (n=5532) | (n=4772) | (n=760) | |||
| Age >65 y | 0.9 [0.8 – 0.9] | 0.03 | 0.9 [0.8 – 0.9] | 0.04 | 1.0 [0.7 – 1.5] | 0.81 |
| Female sex | 1.0 [0.9 – 1.1] | 0.80 | 1.0 [0.9 – 1.1] | 0.80 | 0.9 [0.6 – 1.2] | 0.34 |
| Size, cm | 1.0 [1.0 – 1.0]† | <0.01 | 1.0 [1.0 – 1.0]‡ | <0.01 | 1.0 [1.0 – 1.0] § | 0.23 |
| Size >2 cm | 3.6 [3.1 – 4.1] | < 0.01 | 3.9 [3.3 – 4.7] | < 0.01 | 2.3 [1.6 – 3.2] | 0.01 |
| Size category | ||||||
| <2 cm | ref | ref | ref | |||
| 2–3 cm | 2.2 [1.9 – 2.7] | < 0.01 | 2.4 [2.0 – 3.0] | 0.01 | 1.5 [1.0 – 2.4] | 0.07 |
| >3 cm | 4.4 [3.8 – 5.2] | < 0.01 | 4.9 [4.1 – 5.8] | < 0.01 | 2.7 [1.8 – 3.8] | 0.01 |
1.0011 [1.0004 – 1.0017]
1.0011 [1.0003 – 1.0018]
1.0010 [0.9994–1.0026]
OR, odds ratio
Tumor size and Incidence of LNM
When incidence of LNM were stratified by tumor size, we found that increasing tumor sizes only predicted an increase in nodal involvement for White patients (Table 3). For Black patients, we saw increased incidence of LNM in <2 cm tumors compared to White patients in both the initial (23% vs 5%; p=<0.01) and validation (21% vs 12%; p=<0.01) datasets. The other tumor size categories of 2–3cm and >3cm were similar between cohorts in both datasets. Sub-analysis of tumors <1cm found that Black patients had higher rates of LNM than White patients in the initial (25% [2/8] vs 0% [0/33]; p=<0.01), but not the validation dataset (10% [7/67] vs 9% [31/354]; p=0.66). For tumors 1–2cm we found that Black patients had higher rates of LNM than White patients in both the initial (23% [5/22] vs 7% [8/118]; p=0.02) and validation datasets (25% [7/67] vs 13% [157/1242]; p=<0.01).
Table 3.
Rate of Lymph Node Metastasis by Tumor Size
| Size category | All | White patients | Black patients | p Value |
|---|---|---|---|---|
| Initial dataset | ||||
| <2 cm | 15/181 (8) | 8/151 (5) | 7/30 (23) | < 0.01 |
| 2–3 cm | 18/97 (19) | 15/84 (18) | 3/13 (23) | 0.65 |
| >3 cm | 64/175 (37) | 59/153 (37) | 5/22 (23) | 0.15 |
| Validation dataset | ||||
| <2 cm | 242/1854 (13) | 188/1596 (12) | 54/258 (21) | < 0.01 |
| 2–3 cm | 312/1240 (25) | 267/1084 (25) | 45/156 (29) | 0.26 |
| >3 cm | 970/2438 (40) | 827/2092 (40) | 143/346 (41) | 0.53 |
Data presented as number of lymph node positive/total number of patients with tumor of that size (%)
Disease Free Survival
Of the 453 patients included in the initial dataset available for survival analysis, 51 patients (11%) had recurrent disease (48 White patients and 3 Black patients). For all patients, median DFS for tumors <2 cm, 2–3 cm, and >3 cm was only meet for tumors >3cm (100 months [95% CI 82–100]; p=< 0.01) (see Figure 1). When analyzing by racial cohort, this significance only held for White patients with tumors >3cm (100 months [95% CI 82–100]; p= < 0.01) as seen in Figure 2A. Given only 3 recurrences were reported in Black patients (1 in the 2–3cm cohort and 2 in >3cm cohort), comparisons of DFS in this group cannot be made reliably.
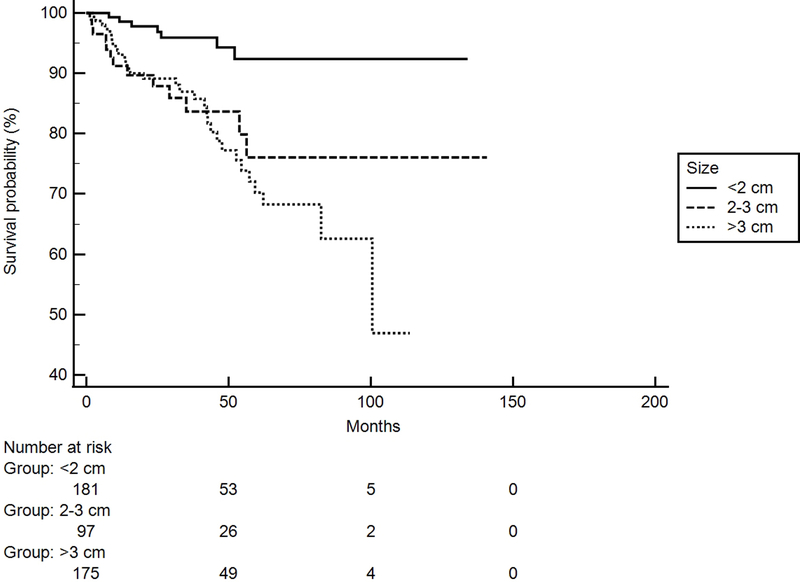
Figure 1.
Kaplan-Meier Curve showing significant difference in disease free survival (DFS) based on size of tumor at presentation in all patients (P < 0.01, n=453).
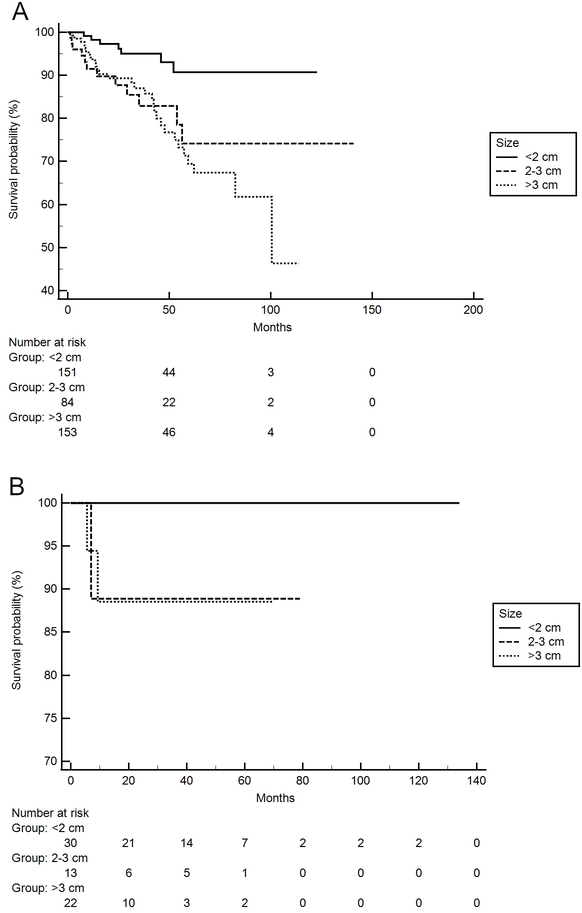
Figure 2.
Kaplan-Meier Curve for DFS based on tumor size for (A)White patients and (B) Black patients. (A) DFS is a significantly different among tumors < 2 cm, between 2–3cm, and >3 cm in White patients (P < 0.01, n=388); (B) DFS was not significantly different in Black patients among tumors < 2 cm, between 2–3cm, and >3 cm (P = 0.21, n=65).
Discussion
Considerations for resection of PNETs include symptom control, tumor size, location, malignant potential, and extent of metastatic disease.(19) Existing guidelines recommend asymptomatic, nonfunctional tumors over 2cm be considered for surgical resection.(6). Multiple studies have validated the prognostic role of tumor size for predicting the risk of LNM. Tumor size, as a continuous variable, was found to be a significant indicator for PNET metastasis.(11) When divided into specific size cohorts, it was shown that the rate of PNET metastasis was significantly lower in PNETs ≤ 2 cm than those > 4 cm as well as tumors between 2–4 cm and those > 4 cm.(12) A 2 cm cut-off was found to be a significant predictive variable for PNET progression in multiple studies, with odds ratios averaging between 5–6.(12, 14) When looking specifically at small, sporadic, nonfunctional PNETs, a study found that the rate of tumor growth is averaged to about 1.5% of the initial tumor size per year with a calculated predicted growth of 0.006 mm/month in PNETs that measure < 2 cm, making patients with small tumors safe candidates for non-operative management. (13) In MEN1 patients, tumors less than 2 cm had lower rate of liver metastasis, which is an important determinant of long-term survival (10) Even functional tumors were found to show decreased malignant potential when smaller than 2 cm.(15) However, none of these studies analyzed the role of tumor size in the context of patient race.
It had been shown that race is an independent prognostic risk factor for long-term survival in gastrointestinal neuroendocrine tumors.(8) Not only was tumor sizes found to be different between non-Hispanic White patients and non-Hispanic Black patients, overall survival and cause-specific survival were significantly higher in non-Hispanic Black patients compared to the non-Hispanic White patients.(20) Specific to PNETs, Black patients had been found to present with more advanced disease with decreased overall survival without surgical resection.(1) Interestingly, a prior analysis of the USNETSG dataset found that while Black patients were more likely to had LNM at resection, if lymph nodes were negative then Black patients had a better 5-year recurrence-free survival compared to White patients.(17) This suggests that earlier resection prior to LNM could have a more significant impact for Black patients, than for White patients. We previously demonstrated that Black patients cared for at our institution presented with larger tumors, but that this did not translate to an increased risk of recurrence.(18) These findings in aggregate suggest that there may be racially distinct associations between tumor size, nodal involvement, and outcomes that warranted further investigation.
This current analysis of the USNETSG and NCDB datasets found that tumor size as a continuous variable did not predict LNM in Black patients with PNETs like it did White patients. Regression analyses showed no association with tumor size to LNM in Black patients except for tumors larger than 3cm in the NCDB dataset, suggesting again that size did not have the same predictive power in this patient group. Alarmingly, we found that significantly more Black patients had lymph node metastases in <2 cm tumors than White patients. This observation may lead providers to recommend resection or more frequent surveillance for smaller tumors in this patient population. Thus, these findings would suggest tumor size is not a reliable criterion for predicting tumor behavior in Black patients. Analysis of DFS in each racial cohort suggests earlier resection in Black patients may impact recurrence, but data is limited.
This study has several limitations. The retrospective nature of this study makes unintentional contributions of bias likely. Individual patients may be represented in both datasets due to the overlap of institutions in the USNETSG and those who submit reports to NCDB. The NCDB does not report WHO grade, but most grade 3 tumors are poorly differentiated so exclusion of these tumors and undifferentiated tumors should focus the analysis on lower WHO grade tumors. The indolent nature of PNETS makes survival analysis difficult even with a median of 3-year follow-up. The low rate of recurrence in Black patients limits meaningful comparison of DFS between racial groups. Finally, DFS is not synonymous with overall survival, thus no conclusions on patient mortality can be derived.(21, 22).
Conclusion
We conclude that current recommendation for surveillance of <2cm PNETs is likely based on a low rate of LNM seen in a predominantly White population. The incidence of LNM in Black patients with <2cm tumors is clinically relevant and concerning. Current guidelines may not be universally applicable and a more aggressive approach towards resection or surveillance in Black patients with small PNETs may be warranted.
Acknowledgement
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC’s NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Research reported in this paper was supported by National Cancer Institute of the National Institutes of Health under award number T32CA229102 and K08CA234209.
Appendix
Members of the US Neuroendocrine Tumor Study Group: Timothy M Pawlik, MD, MPH, PhD, FACS, Division of Surgical Oncology, Ohio State, Columbus, OH; George Poultsides, MD, FACS, Division of Surgical Oncology, Stanford University, Stanford, CA; Clifford S Cho, MD, FACS, Department of Surgery, University of Michigan, Ann Arbor, MI; Kamran Idrees, MD, FACS, Division of Surgical Oncology, Vanderbilt University, Nashville, TN; Flavio G Rocha, MD, FACS, Oregon Health and Science University, Portland, OR; Dr Patrick Varley, MD, Madison, WI; and Dr. James Barrett, MD, Madison WI.
Footnotes
Disclosure Information: Dr Yates receives consulting fees/honorarium from Amgen, QED Therapeutics, and Riptide Biosciences, and owns stock in Riptide Biosciences.
Disclosures outside the scope of this work: Dr Rocha is a paid consultant to Medtronic. All other authors have nothing to disclose.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Members of the US Neuroendocrine Tumor Study Group are listed in the Appendix.
Presented at the Southern Surgical Association 133rd Annual Meeting, Hot Springs, VA, December 2021.
References
- 1.Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017;3(10):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eriksson B, Oberg K, Skogseid B. Neuroendocrine pancreatic tumors. Clinical findings in a prospective study of 84 patients. Acta Oncol. 1989;28(3):373–7. [DOI] [PubMed] [Google Scholar]
- 3.Ehehalt F, Saeger HD, Schmidt CM, Grützmann R. Neuroendocrine tumors of the pancreas. Oncologist. 2009;14(5):456–67. [DOI] [PubMed] [Google Scholar]
- 4.Lahat G, Ben Haim M, Nachmany I, et al. Pancreatic incidentalomas: high rate of potentially malignant tumors. J Am Coll Surg. 2009;209(3):313–9. [DOI] [PubMed] [Google Scholar]
- 5.Carter AM, Kumar N, Herring B, et al. Cdk5 drives formation of heterogeneous pancreatic neuroendocrine tumors. Oncogenesis. 2021;10(12):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falconi M, Eriksson B, Kaltsas G, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103(2):153–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee L, Ito T, Jensen RT. Prognostic and predictive factors on overall survival and surgical outcomes in pancreatic neuroendocrine tumors: recent advances and controversies. Expert Rev Anticancer Ther. 2019;19(12):1029–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai W, Tan Y, Ge W, et al. Pattern and risk factors for distant metastases in gastrointestinal neuroendocrine neoplasms: a population-based study. Cancer Med. 2018;7(6):2699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka M, Heckler M, Mihaljevic AL, et al. Systematic Review and Metaanalysis of Lymph Node Metastases of Resected Pancreatic Neuroendocrine Tumors. Ann Surg Oncol. 2021;28(3):1614–24. [DOI] [PubMed] [Google Scholar]
- 10.Cisco RM, Norton JA. Surgery for gastrinoma. Adv Surg. 2007;41:165–76. [DOI] [PubMed] [Google Scholar]
- 11.Regenet N, Carrere N, Boulanger G, et al. Is the 2-cm size cutoff relevant for small nonfunctioning pancreatic neuroendocrine tumors: A French multicenter study. Surgery. 2016;159(3):901–7. [DOI] [PubMed] [Google Scholar]
- 12.Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery. 2011;150(1):75–82. [DOI] [PubMed] [Google Scholar]
- 13.Gaujoux S, Partelli S, Maire F, et al. Observational study of natural history of small sporadic nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2013;98(12):4784–9. [DOI] [PubMed] [Google Scholar]
- 14.Haynes AB, Deshpande V, Ingkakul T, et al. Implications of incidentally discovered, nonfunctioning pancreatic endocrine tumors: short-term and long-term patient outcomes. Arch Surg. 2011;146(5):534–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonkers YM, Claessen SM, Perren A, et al. DNA copy number status is a powerful predictor of poor survival in endocrine pancreatic tumor patients. Endocr Relat Cancer. 2007;14(3):769–79. [DOI] [PubMed] [Google Scholar]
- 16.Zhou H, Zhang Y, Wei X, et al. Racial disparities in pancreatic neuroendocrine tumors survival: a SEER study. Cancer Med. 2017;6(11):2745–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DePalo DK, Lee RM, Lopez-Aguiar AG, et al. Interaction of race and pathology for neuroendocrine tumors: Epidemiology, natural history, or racial disparity? J Surg Oncol. 2019;120(6):919–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng-Pywell R, Fang A, AlKashash A, et al. Prognostic Impact of Tumor Size on Pancreatic Neuroendocrine Tumor Recurrence May Have Racial Variance. Pancreas. 2021;50(3):347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen RT, Cadiot G, Brandi ML, et al. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: functional pancreatic endocrine tumor syndromes. Neuroendocrinology. 2012;95(2):98–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goksu SY, Ozer M, Beg MS, et al. Racial/Ethnic Disparities and Survival Characteristics in Non-Pancreatic Gastrointestinal Tract Neuroendocrine Tumors. Cancers (Basel). 2020;12(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caplin ME, Pavel M, Ćwikła JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–33. [DOI] [PubMed] [Google Scholar]
- 22.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–63. [DOI] [PubMed] [Google Scholar]