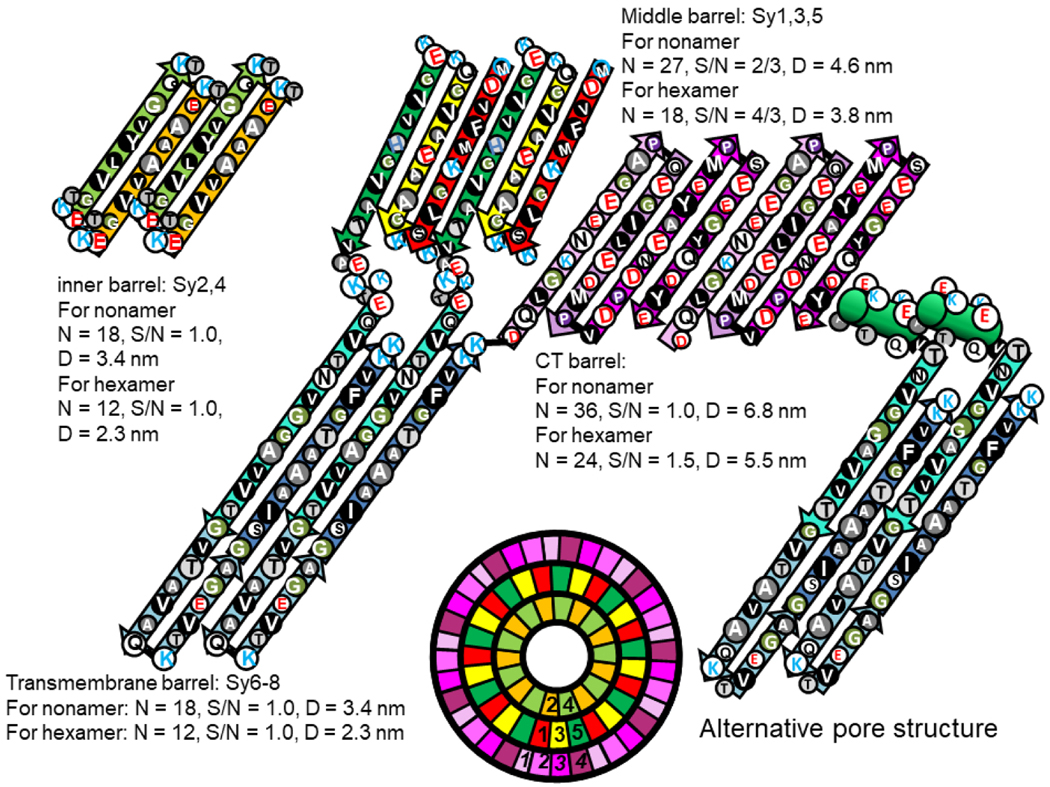
Figure 20.
Flattened representations of two monomers for hexamer and nonamer channels. The wedge schematic on the bottom illustrates relative positions of the α-Syn strands in a cross-section of the nonamer’s soluble domain. Segment numbers of the tentative outer Ct β-barrel are italicized. The Nt domain has a Type 1P conformation with the Sy1,3,5 β-barrel in the middle and the Sy2,4 β-barrel at the core. NAC domains form the transmembrane pore; an alternative model of the Sy5 signature region and the NAC pore is illustrated on the right. Smaller circles represent residues with pore-lining side-chains. The NAC β-hairpins are in series with the inner barrel of the soluble domain but are linked to the Sy5 and Ct1 strands of the two outer barrels. Parameters for each barrel are listed.