FIGURE 7.
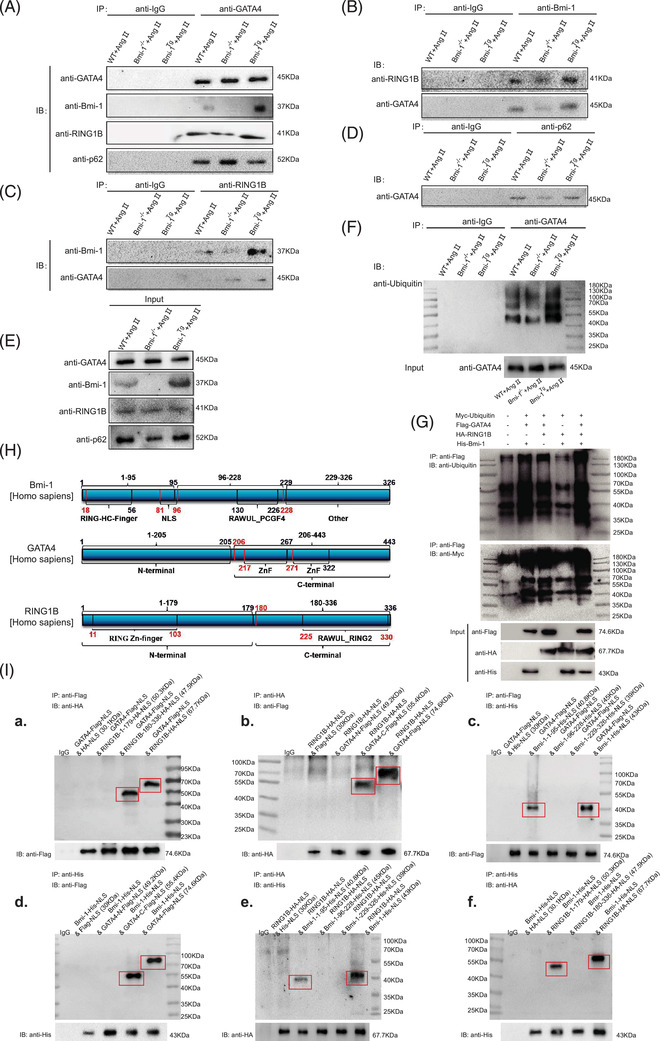
GATA4 binds Bmi‐1‐RING1B and is ubiquitinated, recognised by p62 and degraded by selective autophagy. (A) Myocardial tissue proteins from Ang II‐treated WT, Bmi‐1–/– and Bmi‐1Tg mice were extracted for anti‐GATA4 immunoprecipitation. Western blots were used for detecting GATA4, Bmi‐1, RING1B and p62. (B) The above myocardial tissue proteins were extracted for anti‐Bmi‐1 immunoprecipitation. Western blots were used for detecting RING1B and GATA4. (C) The above myocardial tissue proteins were extracted for anti‐RING1B immunoprecipitation. Western blots were used for detecting Bmi‐1 and GATA4. (D) The above myocardial tissue proteins were extracted for anti‐p62 immunoprecipitation. Western blots were used for detecting GATA4. (E) The above myocardial tissue proteins were extracted for input experiments. Western blots were used for detecting GATA4, Bmi‐1, RING1B and p62. (F) Mouse embryonic cardiomyocytes (MECs) from WT, Bmi‐1–/– and Bmi‐1Tg mice were isolated and cultured from 13.5‐day foetal hearts, and treated with Ang II (5 × 10–6 mol/L for 70 h) to induce hypertrophy, and were extracted for anti‐GATA4 immunoprecipitation. Western blots were used for detecting ubiquitin in immunoprecipitation samples and for detecting GATA4 in input samples. (G) The 293T cells were transfected with full‐length plasmids including Myc‐ubiquitin, Flag‐GATA4, HA‐RING1B and/or His‐Bmi‐1. Cell proteins were extracted for anti‐Flag‐tag immunoprecipitation and detected anti‐ubiquitin and ‐Myc‐tag with Western blots. The input proteins were detected anti‐Flag‐tag, ‐HA‐tag or ‐His‐tag with Western blots. (H) Plasmids of truncated fragments and full length from Bmi‐1 (labelled with His tag), RING1B (labelled with HA tag) and GATA4 (labelled with Flag tag) were co‐transfected in 293T cells. The above cells were extracted for anti‐Flag‐tag, ‐HA‐tag or ‐His‐tag immunoprecipitation. (I) Western blots were used for detecting anti‐HA‐tag, ‐Flag‐tag or ‐His‐tag in immunoprecipitation and input samples
