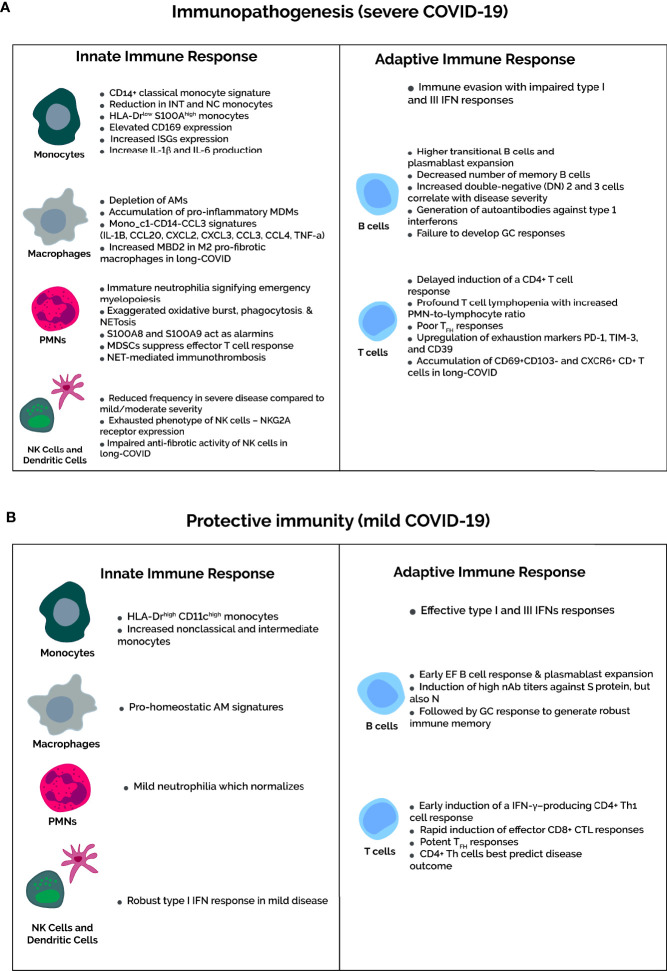
Figure 2.
Severity-dependent immune profiles upon SARS-CoV-2 infection. (A) In severe COVID-19, the immune evasion capabilities of SARS-CoV-2 inhibits IFN responses to delay the recruitment of functional T cells. Consequently, dysfunctional T cell immunity occurs in severe cases, marked by severe lymphopenia and higher expression of T cell exhaustion markers PD-1, TIM-3, and CD39. Not mentioned in the diagram is the increased number of Tregs in severe cases. Concomitantly, amplification of the innate response is characterized by accumulation of classical HLA-DRlowS100Ahigh proinflammatory monocytes and depletion of pro-homeostatic alveolar macrophages. Regarding neutrophils, an immature neutrophilia suggests emergency myelopoiesis, and neutrophil activation markers, such as oxidative burst, phagocytosis, and NETosis, increase in severe COVID-19. In addition, the MDSCs that contribute to the neutrophilia suppress T-cell responses and activate Tregs. Composition rather than quantity of the B cell compartment are altered in severe COVID-19, featuring more antibody-secreting plasmablasts and impaired germinal center responses with a decrease in memory B cells and TFH cells. (B) In contrast, mild COVID-19 infection is associated with recovery of T cell counts and function. The neutrophilia and HLA-DR expression on monocytes normalizes in mild cases. This is due to early induction of IFN responses upon SARS-CoV-2 infection, which elicits an effective and timely T-cell response. B cell compartment modifications are as expected for viral infections, with potent early plasmablast responses and subsequent GC responses to yield long-lived SARS-CoV-2-specific plasma and memory cells.