Figure 5.
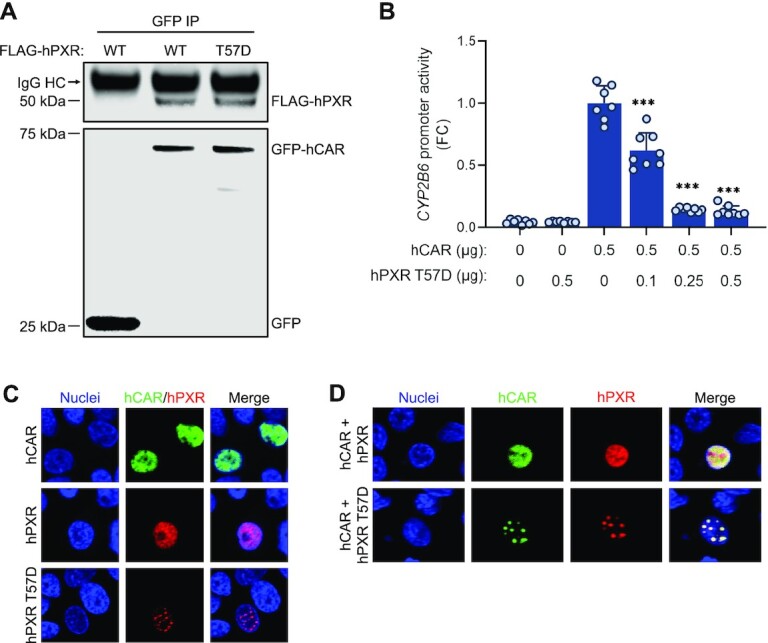
Receptor DNA binding is not required for hPXR–hCAR interaction. (A) hCAR interacts with the DNA binding–defective hPXR T57D mutant. HEK-293 cells were co-transfected with GFP-hCAR and FLAG-hPXR or FLAG-hPXR T57D. The GFP vector was used as a control. Co-IP assays were performed at 48 h post-transfection, followed by immunoblotting with anti-GFP and anti-FLAG. (B) The FLAG-hPXR T57D mutant (hPXR T57D) inhibits hCAR. HepG2 cells were transfected with the CYP2B6-luc reporter plasmid together with the indicated amounts of hCAR and hPXR T57D. CYP2B6 promoter activity was measured at 48 h post-transfection. Empty vector plasmid was used as a control. FC, fold change over cells transfected with hCAR alone. The effect of hPXR T57D on hCAR was determined by comparing its activity to that in cells transfected with hCAR alone. *** P < 0.0005. (C, D) hPXR co-localizes with hCAR. HepG2 cells were transfected with (C) individual GFP-hCAR (hCAR), FLAG-hPXR (hPXR), or the FLAG-hPXR T57D mutant (hPXR T57D) or (D) co-transfected with GFP-hCAR/FLAG-hPXR (hCAR + hPXR) or GFP-hCAR/FLAG-hPXR T57D mutant (hCAR + hPXR T57D). This was followed by immunostaining using antibodies against FLAG (for hPXR) to determine the protein localization at 48 h post-transfection. Nuclei were stained with Vybrant DyeCycle Violet Stain.
