Figure 1.
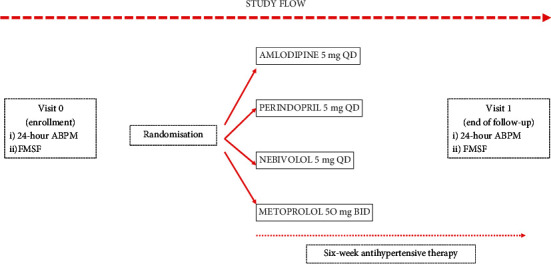
The study protocol. Patients with untreated hypertension were randomised to one of the open-label antihypertensive treatment modes and were prescribed either amlodipine 5 mg once a day (QD) or perindopril 5 mg QD, or nebivolol 5 mg QD or metoprolol 50 mg twice a day (BID). Measurements of the 24-hour ambulatory blood pressure monitoring (ABPM) and the flow-mediated 460 nm skin fluorescence (FMSF) were made at visit 1 before starting the prescribed treatment and at visit 2 after the six-week therapy. Patients were asked to take their morning dose of the prescribed medication on the day of visit 2.
