Figure 2. Development timeline for SGLT-2i and study time blocks.
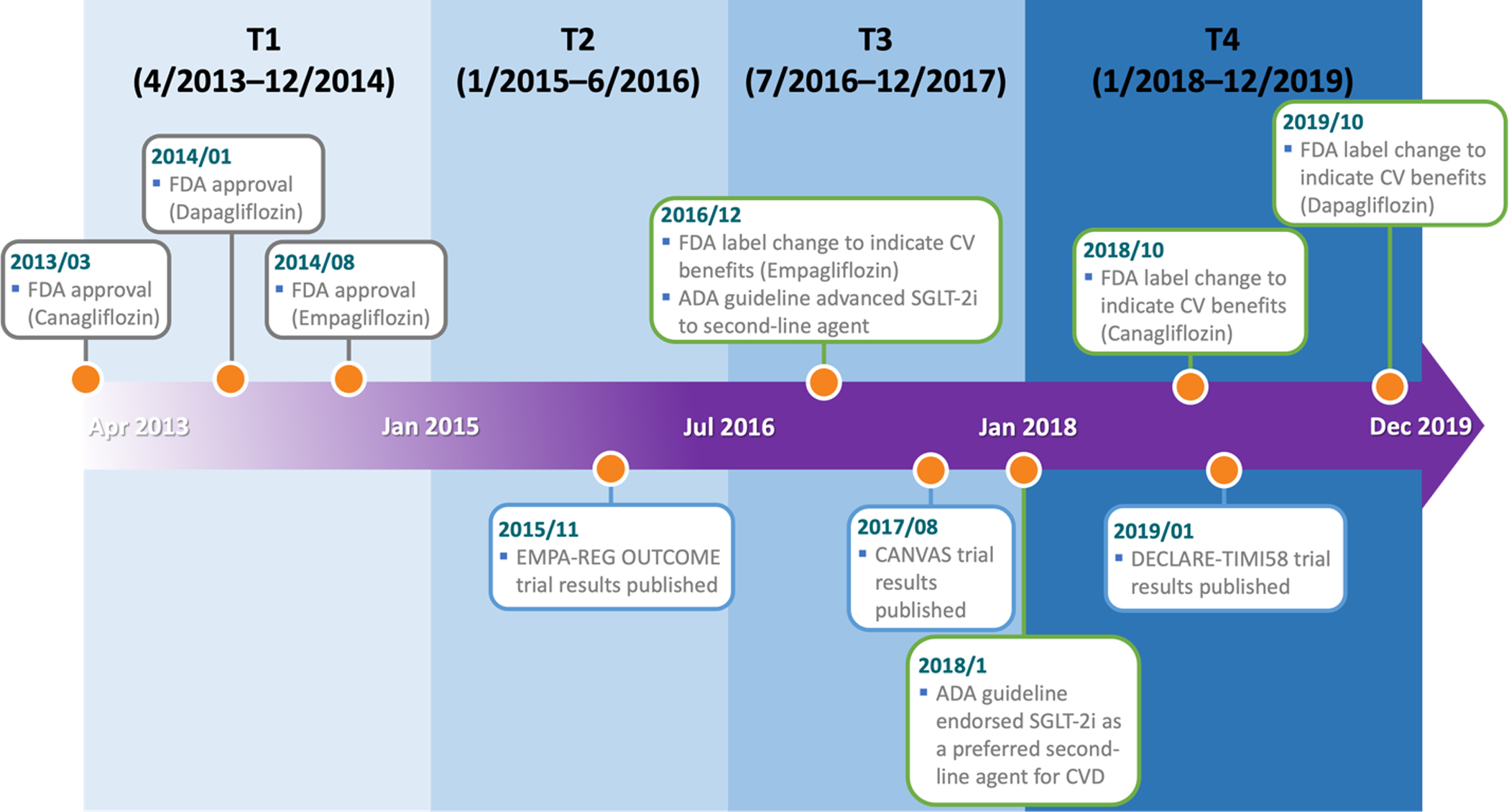
2013/03: FDA approval for canagliflozin (Invokana®) to treat T2D.
2014/01: FDA approval for dapagliflozin (Farxiga®) to treat T2D.
2014/08: FDA approval for empagliflozin (Jardiance®) to treat T2D.
2015/11: Publication of the EMPA-REG OUTCOME trial results for empagliflozin.
2016/12: FDA label change for empagliflozin to indicate cardiovascular benefits.
Advance of SGLT-2i to second-line agent by the American Diabetes Association (ADA).
2017/08: Publication of the CANVAS trial results for canagliflozin.
2018/01: ADA endorsement of SGLT-2i as a preferred second-line agent for patients with CVD.
2018/10: FDA label change for canagliflozin to indicate cardiovascular benefits.
2019/01: Publication of the DECLARE-TIMI 58 trial results for dapagliflozin.
2019/10: FDA label change for dapagliflozin to indicate cardiovascular benefits.
