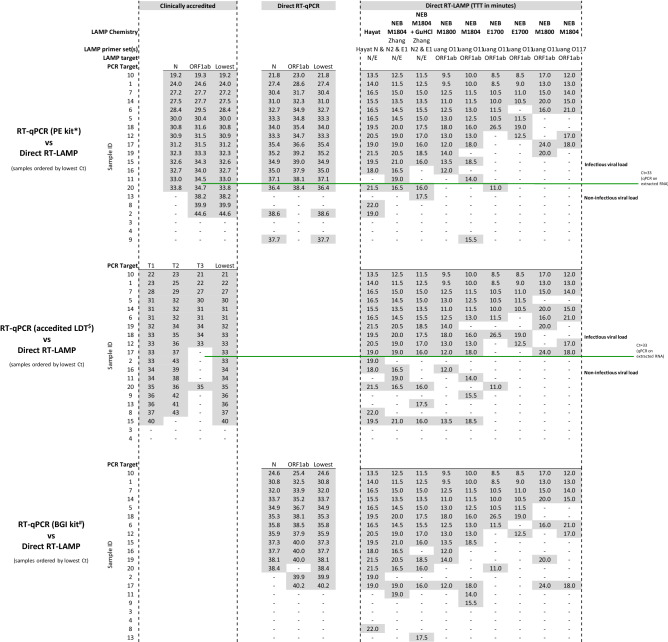
Table 2.
Performance of direct RT-LAMP: performance of direct RT-LAMP compared to RT-PCR.
In this table, RT-LAMP experiments are ordered (left-to-right) from highest to lowest performer. Performance was measured by the highest Ct below which 100% sensitivity was achieved. All NEB chemistries included added Syto9 for detection by fluorometry. All chemistries, except NEB E1700, were colorimetry capable. Colour changes were consistent with fluorometry results. The percentage of reaction volume consisting of sample in RT-LAMP and direct RT-PCR varied from 3.75 to 5%, according to the specified requirements of each test. Purified RNA input of the Perkin Elmer RT-PCR assay kits constituted two-thirds of the total reaction volume, according to the instructions for use.
†'Clinically accredited test' is a diagnostic test/protocol authorized by the Therapeutic Goods Administration (TGA) of Australia and/or FDA under an Emergency Use Authorization (EUA) for detection of SARS-CoV-2. *PE kit: PerkinElmer New Coronavirus Nucleic Acid Detection Kit. Each value shown for Direct-PCR with the PE kit is the mean of two independent heat-inactivation experiments. $Accredited LDT: an accredited laboratory-developed test (LDT) of the Western Australian pathology service laboratory. #BGI kit: BGI Health Real-time fluorescent RT-PCR kit for detecting SARS-CoV-2. TTT: Time to threshold, where threshold is defined as 1.5 × baseline. Dash (–) denotes either no detection of target by 45 cycles of RT-PCR or fluorescence signal did not reach threshold by 30 min in RT-LAMP.